Article Text
Abstract
Introduction Postoperative delirium (POD) is a common complication in elderly patients and is closely associated with delayed recovery, prolonged hospital stays, increased mortality rates and increased medical expenses. Vagus nerve stimulation, a novel technique in the field of neuroscience, has demonstrated remarkable therapeutic potential in improving neurocognitive disorders. However, its applicability in ameliorating neurocognitive dysfunctions that arise during the perioperative period remains unclear. To date, no large prospective, randomised controlled studies have explored the effects of vagus nerve stimulation on POD.
Method and analysis This study is a multicentre, double-blind, parallel, randomised controlled trial. It aims to explore the preventative effects of transcutaneous auricular vagus nerve stimulation on POD in elderly patients who are scheduled for elective surgery at several medical institutions in China from 2024 to 2027. The estimated sample size is 1776, with half of the patients randomly assigned to receive prophylactic standard transauricular auricular vagus nerve stimulation during the perioperative period (allocation ratio 1:1). The primary outcome measure is the incidence of POD within 5 days after surgery.
Ethics and dissemination This study was approved by the Ethics Committee of Beijing Tiantan Hospital, Capital Medical University and adheres to the principles of the Declaration of Helsinki. The protocol was written in accordance with the 2013 Standard Protocol Items: Recommendations for Interventional Trials guidelines. The results of this study will be published in a peer-reviewed journal and presented at national or international conferences.
Trial registration number NCT06421077.
- Delirium
- Cognition
- ANAESTHETICS
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study is a multicentre, prospective, randomised controlled trial to explore the effects of vagus nerve stimulation on postoperative delirium (POD). The study features a rigorous randomising and blinding process, clearly defines inclusion and exclusion criteria, and well-articulated outcome measures, effectively balancing intergroup variable differences.
In accordance with the latest recommendations for POD diagnosis, this study implements a more rigorous and frequent diagnostic approach, in which patients will be assessed two times per day over a period of 5 days. This method is designed to more accurately capture the fluctuating and transient nature of POD, thereby minimising the risk of underdiagnosis to the greatest extent possible.
While anaesthesia type may influence POD incidence, the study does not mandate a standardised anaesthesia protocol to better reflect real-world clinical practice.
The study population is specifically limited to individuals of Chinese ethnicity. Cognitive assessments do not incorporate measures to account for learning effects in this study.
Background
Postoperative delirium (POD) is one of the most common complications in elderly patients after surgery and is associated with prolonged hospital stays, cognitive decline, the need for long-term care and increased medical costs.1–3 The latest report revealed that the incidence of POD in patients over 60 years of age undergoing surgery is as high as 20–50% worldwide.4 5 Preventing POD has become an important public health issue.
Current interventions for POD primarily focus on preoperative education, screening high-risk patients, pharmacological interventions, multimodal postoperative analgesia and the avoidance of certain specific medications.4–6 However, there is a lack of effective and safe measures. Although the use of dexmedetomidine has been proven effective against POD,4 6 7 it also affects the cardiovascular system, potentially causing hypotension and bradycardia. Therefore, the use of dexmedetomidine in elderly patients with comorbid cardiovascular diseases or those at high risk of perioperative stroke requires extreme caution.
The aetiology of POD remains unclear, with the primary theories, including neuroinflammation and neurotransmitter imbalance, potentially induced by surgical trauma and anaesthetic agents, likely playing a significant role.8–10 Surgical stimulation triggers the release of inflammatory mediators and cytokines, including cortisol, C-reactive protein, interleukin (IL)-6, S-100β and IL-8. These factors activate the coagulation system via endothelial tissue, leading to circulatory disturbances and blood‒brain barrier disruption, which exacerbate neuroinflammatory responses. This cascade results in cerebral ischaemia and neuronal apoptosis, contributing to the incidence of POD. Additionally, an imbalance in neurotransmitters such as acetylcholine and dopamine is closely associated with POD. Therefore, rapidly mitigating neuroinflammatory responses and modulating neurotransmitter release in the brain during the perioperative period may prevent and reduce the incidence of POD.
Regulating autonomic function through transcutaneous auricular vagus nerve stimulation (taVNS) to improve cognitive and memory functions, particularly in patients with Alzheimer’s disease, has emerged as a novel therapeutic approach in the field of neurological disorders for decades.11–15 The capacity of taVNS to modulate neuroinflammation and neurotransmitter release has been substantiated in both foundational and clinical studies.16–19 Notably, an animal study demonstrated that taVNS can alleviate sevoflurane-induced cognitive impairments in aged rats by activating basal forebrain cholinergic neurons.20 A subsequent small-scale clinical trial by the same team revealed that taVNS could preemptively address postoperative cognitive delays in elderly patients and diminish the expression of inflammatory cytokines, including AChE, BChE, IL-6, HMGB1 and S100β, in postoperative blood samples, concurrently reducing acetylcholinesterase activity.21 Given the similarity between the effects of taVNS and the primary pathogenic mechanisms implicated in POD, we hypothesise that taVNS may exert a significant preventative effect on POD. There is an imperative for rigorous, prospective, randomised controlled trials to establish the causal relationship between taVNS and POD and to offer a new therapeutic approach for improving cognitive dysfunction in elderly patients in the field of perioperative medicine.
Methods and analysis
Objectives and hypothesis
This study aims to determine the efficacy of taVNS in preventing POD by observing the incidence of POD during the perioperative period in elderly patients. Additionally, this study will assess cognitive function at 90 days after surgery to explore the potential long-term effects of taVNS on cognitive dysfunction in this population. The hypothesis is that the use of taVNS during the perioperative period can reduce the incidence of POD in elderly patients undergoing elective surgery without increasing the incidence of additional complications.
Study design
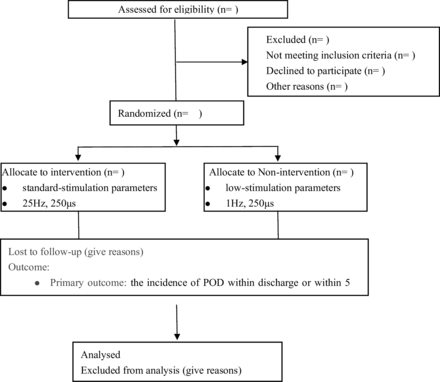
This study is a multicentre, double-blind, parallel, randomised controlled trial with an equal probability of patient enrolment. It consecutively includes elderly patients scheduled for elective surgery at Beijing Tiantan Hospital, Capital Medical University, and several other medical institutions in China from 2024 to 2027. The study design adheres to the Standard Protocol Items: Recommendations for Interventional Trials guidelines. Figure 1 depicts the flowchart for patient enrolment.
Consolidated Standards of Reporting Trials flow diagram for this trial. POD, postoperative delirium.
Population
This study targets elderly patients undergoing elective surgery at designated research institutions over an anticipated period of 3 years. The inclusion criteria for the study are as follows: (1) age≥65 years; (2) anticipated surgical duration ≥2 hours; (3) postoperative hospital stay ≥4 days and (4) provision of signed informed consent (online supplemental file 3). The exclusion criteria are as follows: (1) neurosurgical or cardiac surgery; (2) severe cognitive impairment affecting the assessment of perioperative cognitive function; (3) terminal illness with a life expectancy of less than 3 months; (4) emergency surgery within 6 hours of hospital admission and (5) severe sinus bradycardia, second-degree or higher atrioventricular block, or (6) the presence of an implanted cardiac pacemaker.
Supplemental material
Intervention
In the intervention group, ‘standard-stimulation parameters’ will be employed, with a current stimulation frequency of 25 Hz and a pulse width of 250 μs. In the control group, ‘low-stimulation parameters’ will be used, with a current stimulation frequency of 1 Hz and a pulse width of 250 μs. For both groups, the current intensity will be set to the maximum tolerable level without inducing pain for the patients. The determination of the maximum pain-free tolerable current intensity typically begins at 10 mA; if the subject reports a sensation of tingling within 20 s, the stimulation current intensity is gradually reduced. Conversely, if no tingling is reported, the stimulation intensity is incrementally increased. This maximum pain-free tolerable current intensity, established during the initial stimulation session, will be used for all subsequent sessions.
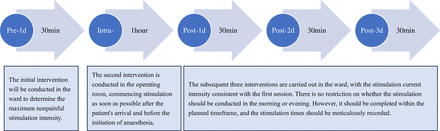
taVNS therapy will be conducted a total of five times. The initial stimulation occurs within 24 hours preoperatively and lasted for 30 min. The second session takes place intraoperatively, with a duration of 1 hour, ideally commencing as soon as the patient enters the operating room and before the surgery begins. The final three sessions are completed postoperatively on days 1–3, with each session lasting for 30 min (figure 2).
Scheduling of five sessions of transcutaneous auricular vagus nerve stimulation interventions. Intra, intraoperatively; Post, postoperatively; Pre, preoperatively.
Randomisation
Once patients are enrolled and confirmed to meet the inclusion and exclusion criteria, they will undergo randomisation via a website-centralised system. The method of randomisation is stratified flexible block randomisation, with a 1:1 allocation ratio for eligible subjects. Stratification criteria are based on the assigned various research centres.
Allocation and blinding
This study will implement blinding for the taVNS stimulation devices to ensure the conduct of a double-blind trial. The taVNS devices used will have fixed, preset frequencies of only 1 Hz and 25 Hz, which are set internally and cannot be adjusted by the investigators or observed through the device display. Investigators can only adjust the output current intensity via the control panel, which displays the setting. Multiple devices with different stimulation frequencies for the two groups are kept by a specific research assistant not involved in this study, who randomly numbers them and correlates the numbered machine information with the central randomisation system in advance. When investigators receive random sequence information, they will directly obtain the machine number required for use from the terminal rather than being informed of groups A or B. For example, assume that machines numbered 1, 4, 5, 6 and 9 have an internal frequency of 25 Hz for the intervention group. When the central randomisation system assigns a patient to the intervention group (hypothetically group A), the investigator will obtain only the random numbers ‘1, 4, 5, 6 or 9’, not the information ‘group A’. The random numbers corresponding to the machines for both groups will only be unblinded at the end of the study to determine group allocation information. The taVNS stimulation devices require regular quality control and calculation (every 2 months) to confirm whether the output current parameters are targeted and timely adjustment as needed. This process is carried out by a specific research assistant in contact with the equipment supplier. Consequently, investigators are only responsible for the implementation of the intervention and are unaware of the stimulation parameters. The subjects in both the intervention and control groups will feel the current stimulation and are equally unaware of their group assignment. Follow-up for primary outcome measures will be completed by an independent follow-up team blinded to group information. Unblinding will be considered only in the event that serious adverse events (AEs) are reported to the principal investigator, the ethics committee and the data safety monitoring board.
Standardised anaesthesia management
The choice of general anaesthesia method, artificial airway selection or opioid, muscle relaxant or regional block technique will be determined by the responsible anaesthesiologist. This study has only established a standard anaesthetic management goal, and regardless of the anaesthetic method chosen by the anaesthesiologist, every effort should be made to achieve the management target values (see online supplemental appendix 1). Furthermore, this study imposes strict restrictions on participating anaesthesiologists, designating specific anaesthesiologists at each centre to perform anaesthesia for this study, thereby reducing the impact of variations in anaesthetic management on the research outcomes.
Supplemental material
Postoperative pain management
Postoperative pain management is conducted in accordance with the routine management protocols of each respective centre, with the autonomy of decision-making vested by the attending anaesthesiologist and the attending surgeon. The objective is to maintain the patient’s pain level at a visual analogue scale score of no more than 3 points.
Data collection and follow-up
According to the data collection plan (table 1), data will be prospectively collected via case report forms (CRFs) in accordance with specific definitions. After hospital discharge, all follow-ups will be scheduled to be completed within 90 days. The need for additional follow-ups will be determined at the discretion of the attending physician. All recorded data must be sourced and submitted to the data management team. For a detailed follow-up plan and content, refer to table 1 and online supplemental appendix 2.
Supplemental material
Schedule of enrolment, intervention and assessment
Outcomes
Primary outcome
This is the incidence of POD at discharge or within 5 days after surgery. POD assessment is conducted via the 3-minute Diagnostic Interview for Confusion Assessment Method (3D-CAM) or Confusion Assessment Method for Intensive Care Unit scale. Assessments are performed two times per day, continuing up to the fifth postoperative day or until discharge. Delirium evaluations are scheduled between 06:00 and 10:00 and between 18:00 and 22:00 daily.
Secondary outcome
Delirium severity: delirium severity is assessed via the 3D-CAM-S scale.
Incidence of delayed cognitive recovery during hospitalisation, as assessed by the Mini-mental State Examination/Montreal Cognitive Assessment.
Incidence of neurocognitive dysfunction at 90 days postoperatively, as assessed by the Abbreviated Mental Test Score.
Degree of decline in activities of daily living at 90 days postoperatively, as assessed by the Instrumental Activities of Daily Living score.
All-cause mortality at 90 days postoperatively.
Proportion of patients requiring higher levels of care postdischarge, specifically long-term care.
Incidence of unplanned admissions to the intensive care unit (ICU) or high-dependence unit.
Length of hospital stay, measured from the initiation of surgery to the actual time of discharge.
Safety outcome measures
The incidence of AEs during hospitalisation will be used as a safety outcome in this study. Any AEs experienced by the subjects will be meticulously documented, irrespective of their association with this study. AEs are classified into anticipated and unanticipated categories. The anticipated AEs include but are not limited to the following: (1) myocardial infarction; (2) cardiac arrest; (3) pulmonary embolism; (4) pulmonary infection; (5) sepsis; (6) surgical site infection; (7) bradycardia; (8) tachycardia; (9) hypotension; (10) hypertension; (11) arrhythmia; (12) hypoxia and (13) coagulation disorders. Definitions for each AE are provided in detail in online supplemental appendix 2.
Sample size calculation
Using PASS15 software (NCSS, LLC, USA) for sample size estimation, data from previous reports indicated that the incidence of POD in patients over 65 years of age undergoing non-cardiac surgery ranges from 12% to 15%.22 23 We aim to reduce POD by at least 30% (15% vs 10%). This study plans to conduct interim analyses at the 25%, 50% and 75% completion of patient follow-ups, adjusting the significance level via the O'Brien‒Fleming method, with the overall significance level controlled at 0.05. With a power of 1-β=80%, the required sample size is 1480. Considering a 20% dropout rate, the anticipated sample size needed to validate a 30% difference is 1776 patients (888 per group). Through interim analyses, the Data Safety Monitoring Board will decide whether to continue the study or terminate it early. If there is a significant discrepancy between the estimated and actual primary outcomes during the study, the final sample size may be appropriately adjusted at the time of the interim analysis.
Interim analysis
Interim analyses of the trial will be conducted after 450, 900 and 1350 subjects (25%, 50% and 75% of the sample size) have completed the 90-day postoperative follow-up. Given the use of the O'Brien‒Fleming method for interim analyses, the corresponding alpha levels for the three interim analyses are α1=0.0001, α2=0.003 and α3=0.018, and a p value <0.044 at the end of the trial is considered statistically significant. All the statistical analyses will be performed via R V.4.4.1. During the interim analysis, the following factors will be considered: recruitment progress of patients, comparability of baselines, sample size assumption on the basis of event rates, attrition, incidence of AEs and the effect of the intervention on the primary outcome. The DSMB will determine whether to continue or discontinue the study on the basis of the interim analyses. For AEs, the DSMB will assess the existing data in conjunction with the anticipated final sample size, and if the threshold is exceeded, the study will be terminated immediately. Regarding the assessment of therapeutic efficacy, if a study has met the efficacy criteria and the required significance level, the study may be concluded early; otherwise, the study will continue. If there is a significant discrepancy between the estimated and actual primary outcomes during the study, adjustments to the sample size may be made at the time of the interim analysis if necessary.
Statistical methods
On the basis of the expected variable frequencies, the incidence of POD, new-onset postoperative neurocognitive dysfunction, transfer to a higher level of care, unplanned ICU admissions and the incidence of AEs will be compared via the χ2 test or Fisher’s exact test. Continuous variables such as hospital stay duration and scale scores will be analysed via t tests if they conform to a normal distribution, and non-parametric tests will be applied if they do not. Logistic regression analysis will be employed to establish a model for independent predictors of POD, evaluating the impact of preoperative conditions, perioperative management, postoperative treatments and complications on POD. In the case of missing data, inverse probability weighting is used, and the worst-case scenario is imputed. In the final analysis, a two-sided p value <0.044 will be considered statistically significant for intergroup differences. Both intention-to-treat (ITT) analysis and per-protocol set analysis principles will be applied in the statistical analysis, with the ITT data set serving as the primary data set for the statistical conclusions.
AE monitoring and reporting
All AEs associated with this study will be meticulously documented and closely monitored until the AE is resolved, the condition stabilises or it can be confirmed that the AE is unrelated to the study. Once an AE occurs, immediate reporting to the research department is needed, along with notification of the principal investigator (PI) of the study to ascertain the severity of the AE and the damage caused. A report must be submitted to the Institutional Review Board within 24 hours as part of the annual report. The PI is responsible for all the reported AEs.
Data Safety Monitoring Board
The study has established a DSMB. The DSMB comprised two anaesthesiology experts and one statistical expert. As an integral part of this research, the DSMB is responsible for providing independent review and assessment of safety data to further protect the interests and safety of the subjects. The primary tasks of the DSMB are to analyse the cumulative data obtained in a phased manner and make recommendations on the basis of the analysis results. All members are required to fulfil the following duties and obligations: understanding the research plan of this study; attending independent monitoring committee meetings; reviewing and signing the clinical trial data safety monitoring committee charter; assessing the safety of the study intervention on the basis of the analysis report from the independent statistician and making corresponding decisions.
Data management
For this trial, an electronic data capture system will be used in conjunction with paper CRFs for the documentation of research data. The coordinating centre will conduct onsite monitoring of source data and perform 50% source data verification for the primary outcome measures. Original data for all data points from the first two subjects enrolled at each centre will be verified, and subsequent monitoring will involve the verification of source data for randomly selected subjects. Study data will be retained for a minimum of 10 years.
Protocol amendments
Any decision to amend the study protocol should be made by the PI. In the event of any modifications to the protocol during the recruitment process (such as changes to inclusion/exclusion criteria, outcome measures and statistical methods), the PI is required to communicate with all departments involved in the study and obtain approval from the Ethics Committee of Beijing Tiantan Hospital, Capital Medical University, prior to the implementation of the amended protocol.
Research progress and schedule
This study received approval from the Ethics Committee of Beijing Tiantan Hospital, Capital Medical University (Ethics Number: KY2024-008-02, 18 February 2024) and was registered on Clinicaltrials.com (NCT 06421077, 10 May 2024). The DSMB was established on 10 June 2024, and the study was subsequently initiated. The first patient was recruited on 20 June 2024. On the basis of the actual medical service situation at our research centre, assuming that 50% of the patients are eligible and consent to participate in this study, we estimate the research period to be 2−3 years. The results of this study will be published in a peer-reviewed journal and presented at domestic or international conferences.
Discussion
In this study, low-frequency stimulation rather than a blank control will be used for the control group. This decision takes into account the frequency dependency of taVNS.24 25 Published research widely acknowledged 20–30 Hz as the safe and effective frequency range.26–28 Studies using fMRI to observe the therapeutic effects of VNS have revealed that higher stimulation frequencies result in more extensive and active brain functional area activation.24 In contrast, activation effects on brain functional areas were limited to frequencies less than 5 Hz compared with 20 Hz.24 Consequently, some studies have adopted 1 Hz as the control group and 25 Hz as the intervention group for taVNS, with both groups demonstrating significant differences.26 28 Compared with sham interventions that place electrodes on non-standard stimulation sites such as the earlobe, this approach better facilitates the blinding of researchers. Moreover, sham interventions with electrodes placed on non-standard sites such as the earlobe may still activate the vagus nerve due to current diffusion under high-intensity electricity.29 Therefore, this study opts for a 1 Hz stimulation frequency as the control group. Pulse width also significantly affects the efficacy of taVNS. Previous studies have indicated that a pulse width of 250 μs may be the optimal stimulation duration. Similarly, this study employed a pulse width of 250 μs.30
POD typically occurs within 1 week after surgery, with some patients with hypoactive delirium often exhibiting lethargy and silence, which are frequently overlooked in clinical settings. Therefore, this study employs a more sensitive 3D-CAM scale for the diagnosis of POD and, in accordance with the latest recommendations for POD diagnosis,5 6 10 31 extends the follow-up period to 5 days postoperatively, with two follow-ups per day, accurately capturing the fluctuating and transient nature of POD and increasing the successful diagnosis rate of POD.
Compared with other similar studies, this study is a multicentre prospective randomised controlled study, with a larger sample size, stricter blinding design and a greater variety of surgical types observed. In addition, the design incorporates stringent randomisation, clear inclusion and exclusion criteria, unified anaesthetic management goals and well-defined outcome measures. However, the study design has several limitations. First, the type of anaesthesia may have a certain impact on the incidence of delirium; for example, inhaled anaesthetics might increase the occurrence of POD, and strictly limiting it to total intravenous anaesthesia might be more conducive to interpreting the study results. This study designates only specialised anaesthesiologists without restricting them to their preferred anaesthesia methods. However, limiting to a specific type of anaesthesia is impractical in clinical settings. No medical institution uses only one type of anaesthesia, especially in China, where many anaesthesiologists prefer a combined intravenous-inhalational approach. Strict limitations could reduce the generalisability of the study’s findings. Second, the study population is limited to the Chinese population. The incidence of POD varies among different ethnic groups, and the efficacy of taVNS may differ accordingly. Third, in the assessment of cognition, there is potential for a learning effect among patients, which has not been specifically addressed with professional interventions. However, cognitive evaluation serves as a secondary outcome measure intended to lay the groundwork for future research endeavours. The primary objective of this study is to elucidate the relationship between taVNS and POD. Introducing additional research procedures could complicate the study.
Patient and public involvement
In the design of this study, there was no direct consultation with patients or the public in the formulation of research questions or outcome measurements. Patients were not involved in the design, recruitment, or clinical implementation of this research. Following the study’s conclusion, the results will be reported in written form. The final research outcomes will also be communicated to all study participants in the manner they are most accustomed to.
Ethics statements
Patient consent for publication
Acknowledgments
The authors thank all those who contributed to the preparation of this protocol.
References
Footnotes
YW and XH contributed equally.
Contributors RH conceived the primary idea of the study and is the principal investigator and guarantor. YW, XH, TW, XW, KZ, FL, MJ, BW and HL executed the study. AW performed the statistical analysis. YW and XH wrote the first draft of the manuscript. All the authors revised this draft and approved the final version.
Funding This work was supported by the Clinical Medicine Development of Special Funding Support (ZYLX201708; DFL20180502) and the Capital Health Research and Development of Special (SF2024-2-2046).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.