Article Text
Abstract
Objectives This study aimed to evaluate survival outcomes and identify key mortality predictors among patients with breast cancer in Ethiopia.
Study design A systematic review and meta-analysis.
Study participants The study used 11 primary studies, involving a total of 4131 participants.
Data sources We searched PubMed, Embase, Web of Science, Scopus and Google Scholar until 7 March 2025, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Eligibility criteria for selecting studies All observational studies that had reported the survival status and/or at least one predictor of mortality of women patients with breast cancer were considered.
Data extraction and synthesis Three independent reviewers (HA, HKN and DGA) used a structured data extraction form to extract the data. To compute the pooled survival and mortality rates, the survival rates at different observation periods and the mortality rates reported in the included studies were extracted.
Results Eleven studies were analysed. All studies were of good quality based on Newcastle-Ottawa Scale. However, heterogeneity was high (I² = 98.2%, p=0.00). Funnel plots showed significant publication bias. The Grading of Recommendations, Assessment, Development, and Evaluations assessment indicated moderate certainty for mortality rates and predictors, limited by heterogeneity and regional data gaps. The pooled mortality rate was 36% (95% CI: 25% to 46%). The survival rates at 1, 3 and 5 years were 85% (95% CI: 75% to 96%), 66% (95% CI: 48% to 84%) and 22% (95% CI: 1% to 43%), respectively. Key mortality predictors included advanced clinical stage (Adjusted Hazard Ratio (AHR): 4.14; CI: 2.53 to 6.78), rural residence (AHR: 1.65; 95% CI: 1.27 to 2.14), positive lymph node status (AHR: 2.85; 95% CI: 1.50 to 5.44), no hormonal therapy (AHR: 2.02; 95% CI: 1.59 to 2.56), histologic grade III (AHR: 1.76; 95% CI: 1.29 to 2.41), hormone receptor negativity (AHR: 1.54; 95% CI: 1.05 to 2.25) and comorbidities (AHR: 2.24; 95% CI: 1.41 to 3.56).
Conclusion Breast cancer in Ethiopia poses a high mortality rate primarily due to late-stage diagnosis, rural residency, histologic grade III, positive lymph node status and comorbidities. To improve survival outcomes, it is crucial to expand access to early screening, particularly in rural areas, implement comprehensive treatment protocols and strengthen healthcare infrastructure to address these critical factors.
PROSPERO registration number CRD42024575074.
- Ethiopia
- Health & safety
- Health Surveys
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. Extracted data are available on request to the corresponding author.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This systematic review and meta-analysis represent a national estimation conducted in Ethiopia.
However, it may lack full national representativeness, as no data were available from the Benishangul Gumuz, Afar, Gambella, Somalia, Dire Dawa and Harar regions.
Additionally, we were unable to compare the impact of different treatment modalities on breast cancer mortality due to the lack of available data.
Introduction
Breast cancer is a leading cause of cancer-related morbidity and mortality in the world, with 2.3 million new cases and 685 000 deaths reported in 2020.1 Despite challenges in diagnostic systems in Africa, breast cancer accounts for one in four diagnosed cancers and one in five cancer deaths among women.2 With an expected 15 244 new cases and 8159 deaths from the disease in 2018, breast cancer is the most common cancer in Ethiopia and the primary cause of cancer-related deaths among women.3 Common risk factors for breast cancer in Ethiopia include a family history of the disease, early menarche, postmenopausal status and never having breastfed.4 Without early detection and treatment, breast cancer can lead to local and distant metastases, ultimately resulting in death.5
The 5-year survival rate for breast cancer varies significantly from country to country due to differences in healthcare systems, early detection programmes, lifestyles and socioeconomic status. For instance, the 5-year survival rate for patients with breast cancer is 84% in the United States, 89.5% in Australia, 81% in Europe,6 69.55% in Iran,7 74% in Vietnam,8 51.07% in Indonesia,9 49.45% in Malaysia9 and 66.1% in India.10And, the 5-year survival rate is 53.4% in South Africa.11
The survival of patients with breast cancer is influenced by various factors, including sociodemographic variables (age, education, financial status and family history), tumour characteristics (size, nodal status, metastasis, stage, location and histologic grade), comorbidities and treatment type.12 13
In Ethiopia, although some primary studies have reported the overall 5-year survival rate, mortality rate and predictors of breast cancer,14–16 there is a lack of comprehensive data on the national survival status and predictors of mortality among patients with breast cancer. Understanding survival outcomes and associated factors is crucial for improving cancer care and guiding evidence-based interventions. Therefore, this systematic review aims to comprehensively evaluate the survival outcomes and identify key predictors of mortality among patients with breast cancer in Ethiopia. By addressing the existing knowledge gaps, this review will provide valuable insights into the current situation and highlight critical factors influencing survival. Furthermore, the findings will be compared with evidence from other settings globally, offering a broader perspective for tailoring healthcare interventions and policy recommendations in the Ethiopian context.
Methods
Study protocol registration and reporting
The protocol for this systematic review and meta-analysis was registered in the PROSPERO database (Registration ID: CRD42024575074), according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.17 At the time of registration, no secondary outcome measures were planned. However, during the review process, the idea of secondary outcome analysis emerged to provide a more comprehensive understanding of the research question. This additional analysis was included to enrich the findings without altering the study’s primary objectives.
Search strategies and sources of information
Searches were conducted in PubMed, Embase, Scopus, Web of Science and Google Scholar databases to identify relevant articles. The search terms used included ‘breast cancer’, ‘breast neoplasm’, ‘breast tumor’, ‘mammary cancer’, ‘outcomes of breast cancer’, ‘breast malignancy’, ‘survival status’, ‘survival rate’, ‘mortality’, ‘death’, ‘mortality rate’, ‘predictors’, ‘determinant’, ‘risk factors’ and ‘Ethiopia’. These terms were combined using the search operators ‘OR’ and ‘AND’ (online supplemental file 1). Cross-references from the bibliographies of selected studies were also reviewed to enhance search coverage. All search records were imported into EndNote X9, where duplicates were eliminated.
Supplemental material
Inclusion and exclusion criteria
All observational studies that had reported the survival status and/or at least one predictor of mortality of women patients with breast cancer were considered. The review included only studies available online until 7 March 2025. Citations without abstracts and/or full text, anonymous reports, editorials, case reports and qualitative studies were excluded from the analysis (online supplemental table S1).
Supplemental material
Data extraction
Three independent reviewers (HA, HKN and DGA) used a structured data extraction form to extract the data. The extraction process was repeated when variations in the extracted data were observed. If discrepancies between the reviewers persisted, another two reviewers (NDB and LM) were involved in resolving them. The data extraction form included the following details: author, year of publication, region, study design, sample size, median survival time, study quality, the survival rate at 1, 3 and 5 years, overall mortality rate and selected predictors of breast cancer mortality.
Quality assessment
The quality of the cohort studies was assessed using the Newcastle-Ottawa Scale (NOS) by two independent reviewers. This tool assesses three key components: the selection of study groups, the comparability of study groups and the ascertainment of exposure or outcome.18 The primary component, focusing on the methodological quality of each study, was rated on a four-star scale. The second component, addressing the comparability of the studies, was graded with up to two stars. The final component, which evaluated the results and statistical analysis of each study, was graded with up to three stars. Overall, the NOS uses three categorical criteria to assign a maximum score of nine points. Studies with scores of ≥7 points were categorised as ‘good’ quality, those scoring 4–6 points as ‘fair’ quality and those with scores of ≤3 points as ‘poor’ quality (online supplemental table S2).
Supplemental material
The quality of the cross-sectional study included in this systematic review and meta-analysis was assessed using the modified Newcastle-Ottawa Quality Assessment Scale for cross-sectional studies.19 This evaluation encompassed various domains, including methodological quality, sample selection, sample size, comparability of groups, outcome assessment and statistical analysis (online supplemental table S3).
Supplemental material
Outcome measurement
The first of the two outcomes of this study is survival status, which refers to whether study participants are alive or dead. This outcome is expressed as the survival rate or mortality rate. The survival rate was computed by multiplying by 100 and dividing the total number of observed patients by the number of living patients at 1, 3 or 5 years of follow-up. Similarly, the mortality rate was calculated by dividing the number of deaths during the follow-up period by the total number of observed patients and multiplying by 100. The secondary outcome of this analysis focused on identifying predictors of mortality among patients with breast cancer in Ethiopia.
Data processing and analysis
The required data were extracted into an Excel spreadsheet and then transferred to the STATA V.17 software for advanced statistical analysis. The general characteristics of the primary studies were summarised in the tables. To compute the pooled survival and mortality rates, the survival rates at different observation periods and the mortality rates reported in the included studies were extracted. Each survival rate’s natural logarithm (LN) was calculated, and the standard errors for both the survival rates and the log-transformed survival rates were computed using Excel. Similarly, for the HR calculation, the HRs and their lower and upper boundary CIs were extracted. The LN of each HR was calculated, and the standard errors of the log-transformed HRs were determined. These calculations, conducted in Excel, provided input data for the meta-analysis to ensure accuracy and consistency. The Cox proportional hazards (PH) model was used for multivariate analysis. This semiparametric model allows for the adjustment of multiple covariates simultaneously, providing HRs with 95% CIs. The PH assumption was checked using the log-log plots—visual inspection of log-log survival curves was conducted to confirm parallelism.
Heterogeneity test, publication bias and certainty evidence
The Cochran Q-test and Higgins’s I² test statistics were calculated to evaluate heterogeneity across all studies. In this context, I² values of 25%, 50% and 75% indicate low, moderate and high heterogeneity, respectively.20 Given the anticipated heterogeneity in breast cancer outcomes across different regions and healthcare settings in Ethiopia, a random-effects model was selected a priori to account for variability between studies.21 This approach provides a more conservative estimate of the overall effect size and is better suited for synthesising data from studies with high heterogeneity. To ensure the robustness of the model, subgroup analysis was done where studies were stratified by region, sample size and publication year to identify potential sources of heterogeneity. To assess publication bias, a funnel plot was generated, and Egger’s test was performed with a significance level of less than 0.05.22 23 To assess and adjust for potential publication bias, we conducted a trim-and-fill analysis using the random-effects model. This method estimates the number of missing studies due to publication bias and recalculates the pooled effect size after inputting these studies.
The certainty of evidence for the pooled estimates of survival rates, mortality rates and predictors of mortality was assessed using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework. The GRADE approach evaluates the certainty of evidence based on five domains: (1) risk of bias, (2) inconsistency, (3) indirectness, (4) imprecision and (5) publication bias. The certainty of evidence is categorised into four levels: high, moderate, low or very low.
Sensitivity analysis
To assess the robustness of the pooled estimates, a leave-one-out sensitivity analysis was conducted. This involved systematically excluding each study one at a time and recalculating the pooled mortality rate to determine whether any single study had a disproportionate influence on the overall results. The analysis was performed using STATA V.17, and the results were compared with the original pooled estimates to evaluate consistency.
Patient and public involvement
None.
Results
Characteristics of included studies
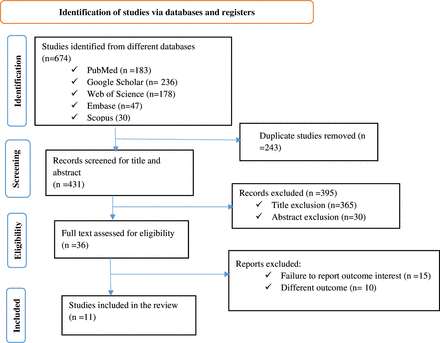
A total of 674 articles were initially retrieved from the PubMed, Embase, Web of Science, Scopus and Google Scholar databases. After removing 243 duplicates, 431 articles remained. Screening titles and abstracts led to the exclusion of 395 articles. The full texts of the remaining 36 articles were assessed, resulting in the exclusion of 25 articles due to different outcomes or failure to report the outcome of interest. Finally, 11 studies met the inclusion criteria and were included in this systematic review and meta-analysis. Figure 1 depicts the retrieval procedure in detail.
PRISMA flow chart for the flow of information through the phases of the systematic review. The chart outlines the process of study identification, screening, eligibility assessment and inclusion. A total of 674 studies were identified from databases (PubMed, Google Scholar, Web of Science, Embase and Scopus). After removing duplicates (n=243), 431 records were screened by title and abstract. Of these, 395 were excluded, and 36 full-text articles were assessed for eligibility. Finally, 11 studies were included in the review. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Based on our assessment, using NOS, all the studies were of good quality. These studies were conducted between 2018 and 2024 and involved a total of 4131 patients diagnosed with breast cancer and started follow-up. Four of the studies were conducted in the Amhara region,14 24–26 three in Addis Ababa,27–29 two in the Southern Nations, Nationalities, and Peoples’ Region (SNNPR),30 31 one in Oromia region32 and one in Tigray region.33 In terms of study design, only one study employed a retrospective cross-sectional approach,29 which was considered only for the determination of the mortality rate. In contrast, the others used a cohort study design. Based on publication years, only three studies were published before 2020.27 29 32 According to the findings of primary studies, the mortality rate among patients with breast cancer ranged from 11.8%33 to 69.6%,31 and all of them were institution-based studies (online supplemental table S4).
Supplemental material
The median age of participants ranged from 39 to 47 years across the studies, with most patients being diagnosed in their early 40s. However, age categorisation varied significantly between studies, limiting the ability to pool age-specific outcomes. A significant proportion of patients were diagnosed at advanced stages (stages III and IV), with reported rates ranging from 56.2% to 83.4% across studies. Rural residency significantly varied across studies ranging from 29.1% to 64%.
Pooled survival status among patients with breast cancer in Ethiopia
A total of 11 studies were analysed to estimate the pooled mortality rate among patients with breast cancer. The heterogeneity among these studies was very high, with an I² value of 98.2% (p=0.00). Using a random-effects model, the pooled mortality rate was calculated to be 36% (95% CI: 25% to 46%) (figure 2). The leave-one-out sensitivity analysis demonstrated that the pooled mortality rate and survival rates were robust to the exclusion of any single study. The pooled mortality rate remained within the range of 34% to 38% (95% CI: 23% to 47%) when each study was excluded, indicating that no single study had an undue influence on the overall estimate.
Forest plot (pooled mortality rate). Forest plot showing the pooled mortality rate among patients with breast cancer in Ethiopia. The plot displays the combined mortality rate estimates from the included studies. Each study is represented by a square, with horizontal lines indicating the 95% CI. The diamond at the bottom represents the overall pooled estimate.
Four studies reported a 1-year survival rate, based on a combined sample size of 1446 patients. The random-effects model analysis showed a significant heterogeneity (I² = 96.99%, p=0.00) and estimated a 1-year survival rate of 85% (95% CI: 75% to 96%). Three studies provided data on the 3-year survival rate, with a total sample size of 1339 patients. The analysis indicated substantial heterogeneity (I² = 98.02%, p=0.00) and estimated a 3-year survival rate of 66% (95% CI: 48% to 84%). Three studies also reported the 5-year survival rate, with a combined sample size of 1519 patients. The random-effects model analysis showed very high heterogeneity (I² = 99.25%, p=0.00) and estimated the 5-year survival rate to be 22% (95% CI: 1% to 43%).
Subgroup analysis of mortality rate
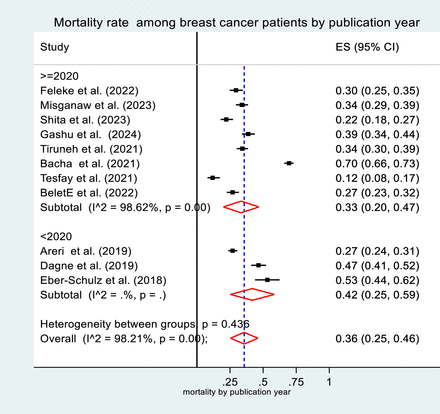
To address the observed heterogeneity in the study (I² = 98.2%), a subgroup analysis of mortality rates was conducted based on region, sample size and year of publication. The analysis by region revealed that the mortality rate among patients with breast cancer was highest in studies conducted in the SNNPR (52%, 95% CI: 49% to 55%), compared with those in the Amhara region (34%, 95% CI: 31% to 38%) and Addis Ababa (33%, 95% CI: 22% to 44%) (figure 3). Additionally, when analysing based on sample size, studies with more than 384 participants reported a higher mortality rate (41%, 95% CI: 21% to 62%) than those with 384 or fewer participants (32%, 95% CI: 23% to 42%) (figure 4). Furthermore, studies published before 2020 showed a higher breast cancer mortality rate (42%, 95% CI: 25% to 59%) compared with those published in 2020 or later (33%, 95% CI: 20% to 49%) (figure 5).
Forest plot (subgroup analysis 1). Forest plot showing the subgroup analysis of mortality rate among patients with breast cancer in Ethiopia. This plot presents the results of a subgroup analysis, stratifying studies by region. Each subgroup is summarised with its pooled estimate and CIs.
Forest plot (subgroup analysis 2). Forest plot showing the subgroup analysis of mortality rate among patients with breast cancer in Ethiopia. This plot provides additional subgroup analysis, comparing mortality rates by sample size. The pooled estimates and CIs are displayed for each subgroup.
Forest plot (subgroup analysis 3). Forest plot showing the subgroup analysis of mortality rate among patients with breast cancer in Ethiopia. This plot illustrates further subgroup analysis by publication year. The results are presented with pooled estimates and 95% CIs.
Publication bias
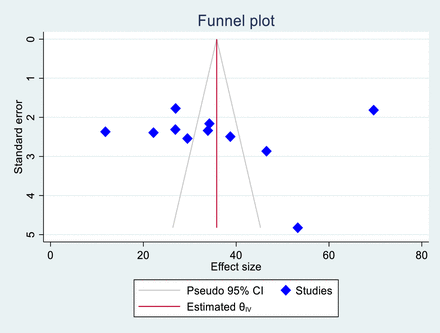
To evaluate publication bias, we used a funnel plot and Egger’s regression test. An uneven distribution in the funnel plot is a subjective indicator of publication bias. Although the objective p value from Egger’s regression test was 0.792, indicating no significant publication bias, we concluded that publication bias was present (figure 6).
Funnel plot (publication bias). Funnel plot showing the results of the publication bias assessment among studies. The funnel plot evaluates potential publication bias in the included studies. Each dot represents a study, plotted by its effect size against its SE. Symmetry around the vertical line suggests a publication bias.
Trim-andfill analysis
In our systematic review, we employed a funnel plot and Egger’s regression test to assess the presence of publication bias. The funnel plot revealed an asymmetrical distribution, which is a visual indicator of potential bias. To mitigate the impact of this bias on our pooled mortality rate, we conducted a trim-and-fill analysis. This method adjusts for publication bias by identifying and ‘trimming’ outlier studies that cause asymmetry in the funnel plot. It then fills the plot with imputed studies, symmetrically opposite to trimmed studies, to reflect a more accurate distribution of the data. As a result of this process, two additional studies were included in our analysis. This adjustment aims to provide a more balanced and unbiased estimate of the pooled effect, enhancing the validity of our review’s conclusions (figure 7).
Funnel plot (trim-and-fill analysis). Funnel plot after trim-and-fill analysis for the pooled mortality rate among patients with breast cancer in Ethiopia. This plot displays the results of the trim-and-fill analysis, which adjusts for potential publication bias. The filled studies are shown as additional dots, and the adjusted pooled estimate is indicated.
Predictors of breast cancer mortality
Data on 10 variables—cancer clinical stage, lymph node status, residence, hormonal therapy, menopausal status, histologic grade at diagnosis, hormone receptor status, comorbidities, tumour size and use of chemotherapy—were extracted into an Excel spreadsheet as two-by-two tables and analysed separately. The analysis identified advanced cancer stage (stages III and IV), rural residence, positive lymph node status, no hormonal therapy, histologic grade III, hormone receptor negativity and comorbidities as significant predictors of breast cancer mortality. Specifically, patients diagnosed at advanced cancer stages (III and IV) had a 4.14 times higher hazard of death compared with those diagnosed at stage I (AHR: 4.14; CI: 2.53 to 6.78). Rural residents experienced a 65% higher hazard of death compared with urban residents (AHR: 1.65; 95% CI: 1.27 to 2.14). Patients with positive lymph node status faced nearly three times the hazard of death compared with those with negative lymph node status (AHR: 2.85; 95% CI: 1.50 to 5.44). Similarly, patients who did not receive hormonal therapy had a twofold higher hazard of death compared with those who received it (AHR: 2.02; 95% CI: 1.59 to 2.56). Patients with negative hormone receptor status had a 54% higher hazard of death compared with those with positive hormone receptor status (AHR: 1.54; 95% CI: 1.05 to 2.25). The hazard of death was 76% higher for patients with histologic grade III tumours compared with those with grade I tumours (AHR: 1.76; 95% CI: 1.29 to 2.41). Additionally, patients with comorbidities experienced a 124% higher hazard of death compared with those without comorbidities (AHR: 2.24; 95% CI: 1.41 to 3.56) (online supplemental table S5). Pooling the effect sizes for some variables was not feasible due to inconsistencies in categorisation across primary studies. For instance, while four studies examined the effect of age on breast cancer mortality, the effect sizes could not be pooled because of inconsistent age categorisations.
Supplemental material
Discussion
In Ethiopia, breast cancer continues to be the most common cancer and the main cause of cancer-related death.34 Moreover, limited resources, insufficient screening programmes, and challenges in early diagnosis and treatment may contribute to rising mortality rates.35 The survival outcomes and predictors of mortality among patients with breast cancer reported in primary studies in Ethiopia show considerable inconsistency. Therefore, this study aimed to determine the pooled survival outcomes and identify predictors of mortality among patients with breast cancer in Ethiopia.
The 1- and 3-year survival rates observed in our study align with findings from similar reviews conducted in various regions around the world.36–40 However, we observed a great variation in a 5-year survival rate between our study and studies done both in developed and developing countries in the world. Thus, the 5-year survival rate in our study is much lower than a study done in the United States, which was reported by Siegel et al.41 The 5-year survival rate for women with breast cancer in developing countries, reported in Uganda and in Zimbabwe, was also higher than the rate observed in our study.42 The differences in survival rates among these studies can indeed be attributed to host factors, tumour factors and medical factors, with a significant emphasis on the availability and effectiveness of screening programmes, early detection and access to modern medical care.37 However, the higher survival rates observed in American and European countries compared with our study are likely due to the impact of screening programmes, early detection and advancements in modern medical care.43 44
This systematic review and meta-analysis revealed that one in three patients with breast cancer succumb to the disease. This mortality rate exceeds the national average death rate for cervical cancer45 and lung cancer46 but is lower than the mortality rate for colorectal cancer.47
In this systematic review, we have identified several critical factors that significantly contribute to breast cancer mortality. These include advanced cancer stage at diagnosis, rural residence, positive lymph node status, no hormonal therapy, histologic grade III, hormone receptor negativity and the presence of comorbidities. In the discussion that follows, we will delve into the implications of these predictors, exploring how each factor individually and collectively influences mortality outcomes in patients with breast cancer.
The prevalence of advanced-stage breast cancer diagnosis among patients in Ethiopia is significantly high.48 According to our review, this prevalent factor increases the hazard of death by fourfold among patients with breast cancer. This association was also observed in previous studies conducted in Hawaii, the United States, Nigeria and Uganda49–53; more importantly, Ferlay J et al pointed out that the prognosis of breast cancer is much better when the disease is detected early, increasing a 5-year survival rate by about two times for localised cases. However, this rate drops drastically to around 25% for cases where the disease has metastasised.54 This information emphasises the importance of early detection and timely intervention in improving survival rates for patients with breast cancer.
Our findings also revealed that patients with breast cancer in rural areas have a higher mortality rate compared with those in urban areas. This outcome is consistent with studies conducted across different regions.55 The higher mortality hazard can be attributed to lower levels of health awareness in rural communities. Moreover, even those who were aware of their condition often faced challenges accessing healthcare services due to limited resources in local hospitals. Supporting this, a study found that women living in rural areas had significantly lower odds of receiving different treatment modalities like surgery, radiation, and surgery with radiation.56
Patients with positive lymph node status faced an increased hazard of death compared with those without. This result was consistently observed in multiple studies conducted across various countries.57 58 This could be due to the result of a higher recurrence rate that is linked to a worse survival rate.59
This review also highlights a significant finding: women diagnosed with histologic grade III breast cancer faced a mortality rate that was 76% higher than those diagnosed with grade I. This finding aligns with previous studies conducted in various Asian countries,60–62 which have similarly reported poorer outcomes for patients with higher grade tumours. The reason behind this could be attributed to the aggressive nature of high-grade cancer cells, which are typically more invasive and linked to a worse prognosis.63
Moreover, the review underscores the strong association between comorbidities and the increased hazard of mortality in patients with breast cancer. This finding is consistent with earlier research from different countries.64–66 The increased vulnerability to treatment toxicity, possibly due to the physiological disturbance of patients with existing comorbid conditions, may explain this correlation.67 Additionally, the presence of comorbidities may influence the cancer’s morphology, histology, differentiation and proliferation status,68 further complicating the disease and its treatment outcomes.
The review identified no hormone therapy as another significant predictor of mortality, revealing that individuals who underwent hormone therapy had a 100% lower risk of death compared with those who did not receive such treatment. These findings align with previous research conducted across various continents.8 61 69 70 Based on this, our review also revealed that the hazard of mortality was significantly higher in patients with hormone receptor-negative tumours compared with those with hormone receptor-positive tumours. This finding aligns with the results of previous studies.71 72 One possible explanation for this disparity is that women with hormone receptor-positive tumours tend to present with more favourable clinical characteristics. Specifically, they are more likely to have early-stage tumours, exhibit moderate differentiation, have negative lymph node status and achieve clear deep surgical margins.73 74 These factors contribute to better overall prognosis and lower mortality risk in hormone receptor-positive patients compared with their hormone receptor-negative counterparts.
This review has several limitations. The high heterogeneity among studies, likely due to variations in sample size, geographic location and healthcare quality, may affect the pooled estimates and generalisability of results. While a random-effects model was used, the wide CIs suggest cautious interpretation. Although Egger’s test showed no significant publication bias, funnel plot asymmetry indicates potential unpublished studies, possibly overestimating mortality rates. Data gaps from regions like Benishangul Gumuz, Afar and Gambella limit national representativeness, as outcomes may vary due to differences in healthcare access and socioeconomic factors. Variability in study quality, inconsistent categorisation of variables (eg, age and tumour size) and lack of data on treatment modalities further constrain the analysis. Despite these limitations, the findings underscore the need for improved early detection, standardised data collection and future research to explore treatment impacts and include underrepresented regions.
Conclusion
Breast cancer remains a significant health challenge in Ethiopia, characterised by high mortality rates largely due to late-stage diagnoses. The findings of this review underscore the urgent need for targeted interventions to improve early detection and treatment of breast cancer, particularly in rural areas of Ethiopia. Given the higher mortality rates observed among rural residents, it is crucial to implement community-based screening programmes that leverage mobile health units and community health workers to reach underserved populations. These programmes should focus on raising awareness about breast cancer symptoms, the importance of early diagnosis and the availability of treatment options. Additionally, training local healthcare providers in rural areas to perform clinical breast examinations and refer suspected cases to specialised centres could significantly reduce delays in diagnosis. Strengthening referral systems between rural health facilities and urban cancer treatment centres, coupled with financial support for transportation and treatment costs, could further improve access to timely and effective care. Public health campaigns should also address cultural barriers and stigma associated with breast cancer, encouraging women to seek medical attention at the earliest signs of the disease.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. Extracted data are available on request to the corresponding author.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
Acknowledgments
We extend our gratitude to the authors of the articles included in this study.
References
Footnotes
X @dagnewgetnet5@gmail.com, @Hab Hab
Contributors The authors have made substantial contributions to this study. HA formulated the research question, drafted the initial manuscript, designed the search strategy, revised and approved the final version of the article. HKN and DGA refined the database search strategy, developed the data extraction form and approved the final version of the article. LM and NDB evaluated the data extraction form and approved the finalised version of the article. HA is the guarantor of this study.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.