Article Text
Abstract
Objectives To assess the upstream pharmaceutical supply chains of 10 high-use pharmaceuticals to detect vulnerabilities that may increase the risk of medicine shortages.
Design Cohort study.
Setting Dutch outpatient setting in 2022.
Participants A total of 407 authorised medicinal products for 10 pharmaceutical substances with the largest number of outpatients.
Main outcome measures The diversity of active pharmaceutical ingredient (API) and finished pharmaceutical product (FPP) manufacturers, their geographic locations and the interdependencies between these manufacturers and marketing authorisation holders (MAHs).
Results For the 407 authorised medicinal products, 50 of the 90 API manufacturing sites were in Asia, and 38 were in Europe. For five pharmaceutical substances, most of the API sites were located outside Europe. Of the 128 FPP manufacturing sites, 94 were in Europe and 31 in Asia. For all 10 substances, at least 47% of FPP sites were located in Europe. API manufacturing for 122 of the 407 products (30%) was entirely performed outside Europe, and FPP manufacturing for 66 of the 407 products (16%). For four substances, more than half of the products depended on API manufacturing outside Europe. The number of distinct API and FPP manufacturing sites per substance was at least four. For amoxicillin, 16 of the 32 products (50%) entirely depended on one and the same API site. For omeprazole, 39 of the 85 products (46%) entirely depended on one and the same FPP site. MAHs applied dual sourcing for API and FPP manufacturing for 61 (15%) of the authorised medicinal products. For three pharmaceutical substances, none of the authorised medicinal products listed at least two API and FPP manufacturing sites.
Conclusion Our study of the supply chains of high-use pharmaceutical substances indicates the need for a granular assessment of the interdependencies between MAHs, API and FPP manufacturers to identify upstream supply chain vulnerabilities.
- Health policy
- PUBLIC HEALTH
- Pharmacists
- Medicine
Data availability statement
No additional data available, since data is related manufacturing locations of specific medicinal products and manufacturers, which is confidential information.Access to the internal database of the Dutch Medicines Evaluation Board (MEB) was granted to DJP as part of her joint PhD trajectory with involvement of the MEB, Royal Dutch Pharmacists Association (KNMP) and University Utrecht. Conflict of interest and a confidentiality agreement was signed by DJP. Data on individual authorised medicinal products, manufacturers and MAHs are published in a de-identified manner. The manuscript was checked by the legal department of the MEB for confidential information prior to publishing.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study offers insights into the pharmaceutical supply chains, focussing on the diversity of active pharmaceutical ingredients and finished pharmaceutical product manufacturers and their geographic locations.
The use of the Dutch Medicines Evaluation Board database to analyse the research question is ideal since this is the official source to register this information.
We visualised the 10 upstream pharmaceutical supply chains using Sankey diagrams, illustrating the complex interdependencies among stakeholders.
Our cohort of 10 pharmaceutical substances is unlikely to be representative of all medicines.
Introduction
A continuous supply of quality-assured, safe, effective and affordable medicines is essential for a well-functioning health system.1 Until the beginning of the 21st century, the availability of medicines did not significantly concern high-income countries. Then, discrepancies between supply and demand began to emerge frequently.2–4 Currently, medicine shortages are ‘the new normal’.5 6 The main causes of medicine shortages are related to manufacturing and quality issues,7 8 which are part of the upstream supply chain, from active pharmaceutical ingredient (API) to finished pharmaceutical product (FPP) manufacturing and packaging, rather than the downstream distribution.9 Despite the increased focus on the availability of medicines during the COVID-19 pandemic, shortages further increased post-COVID-19,10–12 underscoring the necessity of addressing the ongoing challenges in the pharmaceutical supply chain.
The pharmaceutical supply chain is complex, involving multiple interdependent stakeholders and relying on a global distribution network. Marketing authorisation holders (MAHs) are responsible for their authorised medicinal products and for the qualification and selection of the involved (number of) manufacturers. MAHs often rely on manufacturers located worldwide to produce APIs and FPPs.13 MAHs may decide to manufacture APIs and FPPs in-house, which offers control over quality, quantity and timelines, thus providing the flexibility to rapidly respond to manufacturing problems requiring expertise and resources. However, manufacturing generic products is increasingly outsourced, enlarging external dependency and introducing complexity into the upstream supply chain.14 A much-raised additional concern is the geographical location and concentration of manufacturing sites, particularly in Asia.15–17 Studies on manufacturing sites and their geographic locations are limited. For the API market in general, we found data regarding the geographic distribution of manufacturers in relation to turnover. According to the researchers, API manufacturing for the European market is predominantly situated in Asia (56%), followed by Western Europe (24%) and North America (12%), with limited contributions from the rest of the world (8%).18
The supply chain may be disturbed by manufacturing issues, natural disasters or geopolitical disputes. Problems with API availability may disrupt FPP manufacturing, impact the marketing of authorised medicinal products by MAHs, prohibit dispensing by the pharmacist, and ultimately restrict patient access. A robust supply chain would prevent a problem occurring at one point in the supply from causing disruptions elsewhere. To enhance supply chain resilience, ensuring supplier diversity is considered crucial.19 20 For a viable market, a supplier base of at least three different API and FPP manufacturers per pharmaceutical substance is considered desirable according to participants at a WHO-convened technical consultation.21 Dual sourcing strategies per authorised medicinal product—establishing two suppliers for a given ingredient or component in a regulatory product dossier—is also a well-known measure.20 22
Supply chain resilience is in the spotlight of global pharmaceutical policies.17 23 The European Commission is analysing the supply chains of medicines on the European Union (EU) list of critical medicines to identify vulnerabilities. Foreseen EU policy measures to strengthen these supply chains include regulatory flexibilities and recommendations to diversify manufacturers and increase Europe’s manufacturing capacity.24–26 Insight into interdependencies among stakeholders—such as the number of API manufacturers supplying FPP manufacturers and the subsequent number of FPP manufacturers supplying different MAHs, along with their geographic locations—can help to identify supply chain vulnerabilities. Although geographic concentration is often reported as an issue, and medicine shortages and pharmaceutical supply chain vulnerabilities have been linked by some researchers,27–30 no studies have specifically analysed the interdependencies among stakeholders in the upstream pharmaceutical supply chain. There is currently also no method that could be translated into public health resilience planning.
This research aimed to assess pharmaceutical supply chains by evaluating the diversity of API and FPP manufacturers, their geographic locations and the interdependencies between MAHs and these manufacturers. We selected 10 pharmaceutical substances with the largest number of outpatients in the Netherlands since supply disruptions of these medicines may affect a significant share of the population.
Methods
Study population and data collection
For this cohort study, 10 pharmaceutical substances with the largest number of patients in the Dutch outpatient setting were chosen because the number of patients is a key element in determining a shortage’s impact.31 As a result of choosing the number of patients (instead of, for example, the total annual volume), all treatments were counted equally, regardless of treatment duration. The 10 high-use pharmaceutical substances in 2021 originated from the database of the Dutch Foundation for Pharmaceutical Statistics,32 33 which contains complete information on the Dutch outpatient setting, including the outpatient pharmacies in hospitals. The pharmaceutical substances were classified according to the WHO’s Anatomical Therapeutic Chemical (ATC) classification system, which classifies active pharmaceutical substances into five hierarchical levels according to the organ or system on which they act and their therapeutic, pharmacological and chemical properties. ATC on the fifth level indicates the active substance, also known as API.34 We included pharmaceutical substances containing one or a fixed combination of two active components.
The medicinal products containing these 10 pharmaceutical substances authorised in the Netherlands, along with their responsible MAHs and the involved API and FPP manufacturers and their geographic locations, were identified using the Dutch Medicines Evaluation Board (MEB)35 database in July 2022. We excluded products for parenteral use, such as solutions for injection or infusion. These products are mainly prescribed in hospital settings and therefore not included in the data from the Dutch Foundation for Pharmaceutical Statistics.
Study outcomes and data analysis
For the authorised medicinal products, a researcher (DJP) obtained distinct MAHs, API and FPP manufacturers, and geographic locations of the manufacturers at the country and continent levels. The geographic locations were limited to API and FPP manufacturing sites because they are more geographically bound than MAHs, and regulatory requirements are involved when changing them.36
We calculated the median number of authorised medicinal products per MAH and the IQR and range. For authorised medicinal products, we plotted the number of API versus FPP manufacturing sites on a bubble chart. For products containing two pharmaceutical substances, we only plotted the manufacturers of the substances with the fewest API manufacturers because it represented the worst case and, thus, greatest overall vulnerability.
We mapped the pharmaceutical supply chains using Sankey diagrams containing nodes and links to visualise stakeholder interdependencies. The nodes represent the API manufacturer, the FPP manufacturer, authorised medicinal products and the MAH, respectively. The links show their interactions. The width of the nodes is proportional to the number of links; a wider node means more interaction with next-stage stakeholders.
Descriptive statistics present the characteristics of authorised medicinal products and manufacturing sites. Graphics were created using Microsoft Office 365 Excel and Adobe Illustrator (Sankey diagrams).
Patient and public involvement
Neither patients nor the public were involved in the conception, design or execution of this study.
Results
The ten pharmaceutical substances with the largest number of patients in the outpatient setting in the Netherlands included two ATC fifth-level substances containing more than two active components. These two substances were replaced by the next two ATC fifth-level substances that met the inclusion criteria. For the selected substances, 407 medicinal products for outpatient use were authorised in the Netherlands in July 2022 (figure 1). In total, 37% of the Dutch population (6.5 million people) received a prescription for one or more of the selected medicinal products (see online supplemental table S1).
Supplemental material
Supplemental material
Selection of pharmaceutical substances with the largest number of patients in the Dutch outpatient setting and the related authorised medicinal products for analysis. ATC, Anatomical Therapeutic Chemical.
The 407 products included authorised off-patent medicinal products predominantly intended for oral use (378; 93%), with a few for inhalation (21; 5%) or rectal use (8; 2%). The number of authorised medicinal products per pharmaceutical substance ranged from 21 for levonorgestrel/ethinylestradiol and salbutamol to 85 products for omeprazole. The 407 authorised medicinal products were the responsibility of 70 distinct MAHs (see online supplemental table S1), and their manufacturing involved 90 distinct API sites and 128 distinct FPP sites. Three manufacturing sites produced APIs as well as FPPs. In our cohort of 407 products, the 70 MAHs were responsible for a median of three (IQR: 2–6) authorised medicinal products (range: 1–42). Of the 70 MAHs, 49 had marketing authorisations for products for one of the ten pharmaceutical substances. Three MAHs had marketing authorisations for products for nine of the ten pharmaceutical substances.
API manufacturing
The 90 API manufacturers for the 407 authorised medicinal products were mostly located in Asia (50; 55%), a large minority in Europe (38; 42%) and rarely in the Americas (2; 3%) (see table 1 and online supplemental figure S1). The number of distinct API manufacturing sites per pharmaceutical substance ranged from four for levonorgestrel/ethinylestradiol to 17 for pantoprazole. For desloratadine, diclofenac, metoprolol, pantoprazole and simvastatin, more than half of the API manufacturing sites were located outside Europe (54%–82%). For amoxicillin and omeprazole, the API sites were equally divided outside and within Europe. For the remaining three substances, a minority of the sites were located outside Europe (range: 0%–40%), thus the majority were located within Europe (60%–100%). For all pharmaceutical substances, at least two API manufacturing sites were located in Europe.
Supplemental material
Number and geographic location (continent) of active pharmaceutical ingredient (API) manufacturing sites and related medicinal products
Among the 407 authorised medicinal products, 122 entirely relied on APIs manufactured outside of Europe (table 1). For four substances (desloratadine, diclofenac, pantoprazole and simvastatin), the authorised medicinal products predominantly relied on only API manufacturing sites outside of Europe (range: 52%–66%). For colecalciferol, metoprolol and omeprazole, the API manufacturing sites were outside and within Europe. For the remaining three substances, the minority of the products relied on API sites outside Europe (range: 0%–25%). The number of authorised medicinal products per substance specified per country of the manufacturing site is displayed in online supplemental figure S1.
FPP manufacturing
The 128 FPP manufacturing sites for the 407 authorised medicinal products were mainly situated in Europe (94; 74%), to a lesser extent in Asia (31; 24%) and rarely in the Americas (3; 2%) (see table 2 and online supplemental figure S1). The number of distinct FPP manufacturing sites per pharmaceutical substance ranged from seven for levonorgestrel/ethinylestradiol to 23 for simvastatin. For pantoprazole, a small majority (53%) of FPP sites were located outside Europe and for amoxicillin the FPP sites were equally divided outside and within Europe. For the other eight substances, the minority of FPP sites were located outside Europe (range: 0%–48%). For all ten pharmaceutical substances, at least five FPP manufacturing sites were present in Europe.
Number and geographic location (continent) of finished pharmaceutical product (FPP) manufacturing sites and related medicinal products
Of the related authorised medicinal products, 66 of the 407 (16%) were entirely manufactured outside Europe (table 2). For all substances, the minority of the authorised medicinal products relied solely on FPP manufacturing outside Europe (range: 0%–38%). The number of authorised medicinal products per substance specified per country of the manufacturing site is displayed in online supplemental figure S1.
Diversity of manufacturers
The regulatory dossiers of 346 of the 407 products (85%) listed either one API manufacturing and multiple FPP sites (83; 20%), one FPP manufacturing and multiple API sites (157; 39%) or one API and one FPP site (106; 26%) (figure 2). For 61 authorised medicinal products (15%), at least two API and FPP sites were listed. For four substances, most authorised medicinal products (69%–78%) relied on one API manufacturing site. For eight substances, more than half of the authorised medicinal products (52%–95%) relied on one FPP manufacturing site (see online supplemental table S2). For amoxicillin, colecalciferol and levonorgestrel/ethinylestradiol, none of the authorised products listed two or more manufacturing sites for the APIs and FPPs (see online supplemental figure S2). For simvastatin and pantoprazole, most of the authorised medicinal products (both 61%) listed at least two manufacturing sites for the APIs and FPPs.
Supplemental material
Supplemental material
Number of authorised medicinal products with the corresponding number of manufacturing sites of active pharmaceutical ingredients (APIs) and finished pharmaceutical products (FPPs) according to the Dutch Medicines Evaluation Board database (n=407). Red=one manufacturing site for APIs and FPPs; orange=one manufacturing site for APIs or FPPs; green=at least two manufacturing sites for APIs and FPPs.
Interdependency
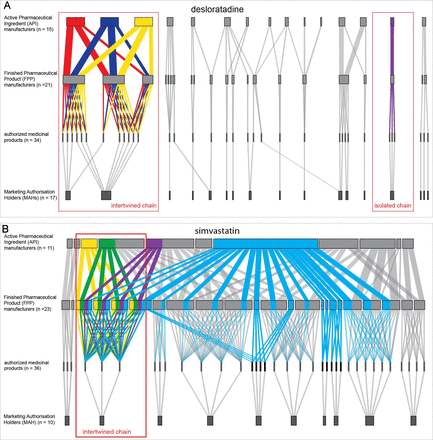
The flow of the 10 upstream pharmaceutical supply chains is illustrated in Sankey diagrams showing the journey from API manufacturer to FPP manufacturer, ending in an authorised medicinal product under the responsibility of an MAH (see online supplemental figure S3). We identified two main patterns of supply chains, the ‘isolated chain’ and the ‘intertwined chain’, for example, as depicted in the diagrams of desloratadine (figure 3A) and simvastatin (figure 3B), respectively. The isolated chain involves (in extremis) one MAH depending on one API and one FPP manufacturer. The intertwined chain consists of multiple API and FPP manufacturers serving multiple MAHs.
Supplemental material
Exemplary supply chains for desloratadine (A) and simvastatin (B).
Of the 70 distinct MAHs, 11 (16%) depended on one API manufacturer and one FPP site (isolated chain), 14 (20%) on one API manufacturer and multiple FPP sites, 20 (28%) on multiple API sites and one FPP manufacturer and 25 (36%) on multiple API and FPP sites (intertwined chain). When an MAH relied on several manufacturers, the same combinations of API and FPP manufacturers (intertwined chain, figure 3A,B) occurred.
Simvastatin was remarkable because 29 of the 36 (81%) authorised medicinal products listed the same API manufacturer (figure 3B; light blue). On closer examination, only nine of these products (25%) entirely depended on this manufacturer. A further analysis of the manufacturer dependencies for all substances showed that amoxicillin was notable for 50% of authorised products relying on one and the same API manufacturer, and omeprazole was notable for 46% of authorised products relying on one and the same FPP manufacturer (see online supplemental table S2).
Discussion
Principal findings
This study on the upstream pharmaceutical supply chains of 10 high-use pharmaceuticals unravelled several overall existing concerns. For these substances, a significant proportion of the API and FPP manufacturing sites were located in Europe, and an even higher proportion of the related authorised medicinal products listed an API or FPP manufacturing site in Europe. All 10 substances had a supplier base exceeding at least three different API and FPP manufacturers, which is desirable for a viable market.21 Dual sourcing for API and FPP manufacturing, however, was present for a minority of the authorised medicinal products.
The dependency on API and FPP manufacturing sites in Asia15 16 was less pronounced than expected. For the 10 substances, 42% and 73% of the API and FPP manufacturing sites, respectively, were located in Europe and were involved in the manufacturing of 70% and 84% of the related authorised medicinal products, respectively. However, for four pharmaceutical substances, more than half of the products (52%–66%) did rely on non-European API manufacturers. For each pharmaceutical substance, at least four different API and seven different FPP manufacturers were listed, thus exceeding a supplier base of at least three manufacturers for APIs and FPPs.21 Nevertheless, some substances were overdependent on one and the same manufacturer, such as amoxicillin (strongly depending on one and the same API manufacturer) and omeprazole (strongly depending on one and the same FPP manufacturer). MAHs had adopted a dual sourcing strategy for API and FPP manufacturing for only 15% of the authorised medicinal products. For the three substances, none of the authorised products listed two or more manufacturing sites for the APIs and FPPs. This limited share of products with dual sourcing for APIs and FPPs and the overdependency on specific manufacturers were serious supply chain vulnerabilities observed in this study.
Comparison with previous research
Studies on API and FPP manufacturers and their geographic locations have been limited. For the API market in general, we found data regarding the geographic distribution of manufacturers in relation to turnover according to suppliers.18 Since the underlying numbers are lacking, the data are difficult to interpret. Recently, the European Commission published the results of the assessment of the supply chain vulnerabilities conducted in 2024 for a first tranche of 11 critical medicines from the Union list.25 Using data collected from both EU member states and MAHs, several aspects were evaluated including diversification and geographic location of manufacturers. The risk thresholds/levels applied in this assessment (eg, high risk with <4 manufacturing sites), suggest that even more substances are at high risk compared with our findings. Similar to our study, this assessment found that MAHs were relatively less dependent on non-EU finished product manufacturers compared with their dependency on non-EU API manufacturers.
A recent study on generic APIs linked their manufacturing characteristics to medicine shortages in the USA.30 This linkage provides an interesting possibility for further research.
Strengths and limitations
The major strength of our study is that we could analyse API as well as FPP manufacturing sites, the responsible MAHs and their interdependencies. Our study showed that an analysis of the geographic location of only manufacturing sites is limited, since the geographic distribution of manufacturing sites differed from the geographic distribution of the sites for the medicinal products. Whereas 42% of the API manufacturing sites were located in Europe, 70% of the authorised medicinal products listed an API manufacturing site in Europe (36% in Europe only and 34% in Europe and another continent). For FPP manufacturing, 73% of the sites were in Europe and 84% of the authorised medicinal products listed a site in Europe. Larger differences were observed at the individual substance level. Our analysis also yielded insights into the implementation of dual sourcing for over 400 authorised medicinal products.
Our study has three limitations. First, our cohort of 10 high-use pharmaceutical substances out of over 13,00020 is unlikely to be representative of all medicines. For example, all of our substances are related to off-patent products. However, diverse supply chains were expected for high-use medicines because they are usually marketed by many MAHs. Also, the manufacturing of these off-patented medicines introduced complexity into the upstream supply chain due to increased outsourcing.37 As these off-patent medicines are often expected to have a relatively robust supply chain, our approach highlights the minimum risks that the supply chain may face. The overdependency on one and the same manufacturer, as observed for some substances in this study, is expected more often for other pharmaceutical substances, such as substances for niche medicines. For these low-use pharmaceutical substances, dual-sourcing may not always be possible, for example, because of a single supplier, or be particularly costly considering the small scale of production.20 26 For the 10 substances in the present study, we also detected different patterns in the supply chains (isolated and intertwined supply chains), but we could not identify an overall sourcing strategy based on our data. Second, the included products were selected based on marketing authorisation in the Netherlands. Although MAHs may have different product portfolios in various countries, similar supply chain patterns are expected for products licenced in other countries in the EU or the European Economic Area (EEA), because most regulatory pathways in this region lead to authorisation in multiple member states or the entire EU or EEA.38 Third, we focused only on API and FPP manufacturers and MAHs. Supply vulnerabilities can also be related to other factors, such as raw material and intermediate manufacturers, packaging sites, wholesalers and distributors. The selected stakeholders are a crucial starting point, since they represent stringently regulated, core entities in the supply chain and information on them could be extracted from a regulatory authority’s database.
Implications for policymakers and clinicians
Our granular analysis of the upstream pharmaceutical supply chains, displayed in Sankey diagrams, better facilitates the identification of supply chain vulnerabilities than numerical metrics. This facilitation will contribute to establishing effective measures to mitigate medicine shortages.
This study provides transparency in the API and FPP manufacturing and related MAHs of 10 high-use pharmaceuticals. Although the information on authorised medicinal products and related MAHs was readily available in the public database of medicine agencies, the specific manufacturing sites were not disclosed. Product-specific information regarding supply chains is closely guarded by the MAHs as trade secrets or confidential commercial information.39 40 Even though authorities have access to information on the specific manufacturing sites, this information is not necessarily available in a format enabling automated processing.20 More transparency and standardised data on the supply chain, such as information on the APIs and FPPs that manufacturers prefer,41 would allow for an improved analysis of vulnerabilities by MAHs or authorities. Various stakeholders have advocated the need for further transparency.17 20
The EU is conducting an analysis of the supply chains for medicines on the EU list of critical medicines to identify vulnerabilities and determine how these can best be addressed.24 However, supply chains for pharmaceutical substances not included on the current EU list of critical medicines, also showed vulnerabilities, such as strong dependency on one and the same FPP manufacturer (omeprazole) and manufacturing sites mainly located outside Europe (simvastatin). Policymakers should not overlook substances that are not indicated as critical at a regional or national level since supply interruptions for non-critical substances may also have a considerable societal impact due to the significant number of patients affected. Our study showed that the supply chains for these 10 substances may be vulnerable due to the lack of dual sourcing, and overdependency on a specific manufacturer.
We acknowledged that our cohort consisted of only 10 pharmaceutical substances. Larger and more systematically differentiated samples (such as substances with established supply shortages) may yield different findings. We encourage future researchers to investigate this topic for complementary insights.
In addition to transparency concerning API and FPP manufacturers and MAHs, an analysis of the relationship between supply chain vulnerabilities and the occurrence of medicine shortages in daily practice could provide further insights to help establish secure, resilient pharmaceutical supply chains.
Conclusion
Our study on the supply chains of high-use generic pharmaceutical substances identified the need for a granular assessment of the interdependencies between API and FPP manufacturers and MAHs to identify upstream supply chain vulnerabilities. Policymakers should direct and amend their policies to effective measures to mitigate medicine shortages. They may also need to acquire a better understanding of the supply chains and its resilience. To aid, the method used in this study could be translated into a tool for public health resilience planning.
Data availability statement
No additional data available, since data is related manufacturing locations of specific medicinal products and manufacturers, which is confidential information.Access to the internal database of the Dutch Medicines Evaluation Board (MEB) was granted to DJP as part of her joint PhD trajectory with involvement of the MEB, Royal Dutch Pharmacists Association (KNMP) and University Utrecht. Conflict of interest and a confidentiality agreement was signed by DJP. Data on individual authorised medicinal products, manufacturers and MAHs are published in a de-identified manner. The manuscript was checked by the legal department of the MEB for confidential information prior to publishing.
Ethics statements
Patient consent for publication
Ethics approval
Ethics approval was not required according to Dutch law, since this study did not involve human subjects.
Acknowledgments
The authors wish to thank the Dutch MEB for access to data in de MEB internal database and Rutger van den Broek (Dutch MEB) for retrieving the data from the internal database.
Footnotes
Contributors All authors conceived and designed the study. DJP performed the first analysis of the data and KN verified the study data. All authors contributed to the interpretation of the results. DJP and KN drafted the manuscript and prepared tables and figures. All authors read and critically reviewed and commented on each draft of the manuscript and approved the final manuscript for submission. DJP is the study guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding This work was supported by the Dutch Medicines Evaluation Board and the Royal Dutch Pharmacists Association. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Disclaimer The views expressed in this article are the personal views of the authors and must not be understood or quoted as being made on behalf of or reflecting the position of the Dutch Medicines Evaluation Board or the Royal Dutch Pharmacists Association.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.