Article Text
Abstract
Objectives To investigate how doctors and self-managing older patients with heart failure (HF) discuss the patients’ potential or ongoing medication adherence problems, and how such discussions evolve as patients transition from hospital to home, with particular focus on: (1) doctors’ communicative actions aimed at addressing patient disclosures of adherence problems and (2) patients’ feedback indicating whether their doctor’s supportive actions were acceptable to them.
Design Exploratory interaction-based observational cohort study. Inductive microanalysis of authentic patient–doctor consultations, audio recorded for each patient at: (1) first ward visit in hospital, (2) discharge visit from hospital and (3) follow-up visit with general practitioner (GP).
Setting Hospital and primary care, Norway (2022–2023).
Participants 25 patients with HF (+65 years) and their attending doctors (23 hospital doctors, 25 GPs).
Results Analysis of 74 consultations revealed that 25 HF patients disclosed 23 practical adherence problems indicating risks of unintentional non-adherence (eg, limited resources to manage medications) and 39 perceptual problems indicating risks of intentional non-adherence (eg, worries, negative experience or stance). Doctors addressed 79% of patients’ disclosures by: (1) exploring the scope of the problem or (2) providing supportive actions to improve patients’ ability or motivation to adhere. We calculated nearly five times higher odds for doctors to address patients’ practical problems to their perceptual problems (OR 4.79, 95% CI 1.25 to 25.83). Unresolved problems included: (1) doctors addressed patients’ disclosures, but patients signalled the supportive actions were unsuitable (37%) and (2) doctors left disclosures unaddressed (21%).
Conclusions In this explorative study, the doctors were more likely to address the patients’ adherence problems associated with unintentional non-adherence risks than those associated with intentional non-adherence risks. Even when doctors attempted to address HF patients’ medication adherence problems, half of the problems remained unresolved, usually because patients indicated that the doctor’s suggestion to improve their situation was against their preference.
- Heart failure
- Medication Adherence
- Clinical Decision-Making
- Patient-Centered Care
- Observational Study
- Hospital to Home Transition
Data availability statement
No data are available. This study uses audio recorded authentic medical consultations. We do not have permission to share these with other researchers.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Heart failure
- Medication Adherence
- Clinical Decision-Making
- Patient-Centered Care
- Observational Study
- Hospital to Home Transition
STRENGTHS AND LIMITATIONS OF THIS STUDY
A detailed and comprehensive description of how often and how doctors respond to heart failure patients’ disclosures indicating risks of medication non-adherence and, in turn, how patients respond to doctors’ supportive actions.
Analysis of authentic medical consultations at three key time points for each patient as they transition from hospital to home.
Participant reactivity to the study situation may have led to more talk about medications and ‘best practice behaviour’.
Limited generalisability to other settings and patient groups.
Introduction
Heart failure (HF) is a chronic, life-threatening condition prevalent among older people.1 2 The global burden is high (estimated to affect 64 million people in 2023) and growing, due to an ageing population.1 The cornerstone of HF management to alleviate symptoms, reduce hospital admissions and improve life expectancy is pharmacotherapy, using a combination of four to five medications.3–5 Older patients with HF often have comorbidities, leading to complex regimens with more than ten medications.6 7 In this patient group, medication adherence is alarmingly low,8 9 thereby limiting therapeutic benefits.10 Patients with HF fail to take their medications as prescribed for several reasons, including not understanding the prognosis and the purpose of their prescriptions, complex medication schedules and experience of adverse effects.11–15 Medication non-adherence can be intentional or unintentional16 17, which emphasises the need for doctors to assess patients’ ability and motivation to take their medications as prescribed.18 Therefore, guidelines recommend that clinicians talk to patients about their medication use to ensure that any treatment decisions are based on current intake of medications.19 20
Although good communication between patients and doctors improves medication adherence21 22, little is known about how patients with HF and their doctors talk about adherence in medical consultations. Indeed, most studies analysing interactions have focused on other patient groups in outpatient settings.23–29 More knowledge is needed about how doctors and patients with HF talk about adherence problems, and how doctors address such problems. Building such knowledge begins with defining these phenomena, identifying and analysing them as they occur in authentic consultations, and deriving implications for enhancing future practice. Due to frequent hospital readmissions in this patient group, longitudinal studies can inform how conversations about adherence problems evolve over time and experience and as patients are cared for by different doctors in hospital and primary care. Ideally, acquired knowledge can inform content and examples for communication skills training aimed at improving patient adherence.
In a previous study, we analysed 74 real-life consultations between 25 self-managing older patients with HF and 48 doctors and found that the patients often disclosed information to their doctors that signalled potential or ongoing medication adherence problems at home.30 The present study built on these identified problem disclosures and aimed to investigate the discussions that emerged from them. Data were the same authentic audio recorded consultations and medical records collected at three time points as patients transitioned from hospital to home. We recognised, defined and counted our phenomena of interest: (1) doctors’ communicative actions aimed at addressing patient disclosures of adherence problems and (2) patients’ feedback to the doctors indicating whether their supportive actions were acceptable to them.
Methods
Overview of study design, participants and data collection
This is an exploratory interaction-based observational cohort study. We followed 25 older patients with HF from their admission to the hospital to their return home and their first follow-up visit with their general practitioner (GP).
Recruitment of study participants (patients, hospital doctors and GPs) and data collection took place from February 2022 to February 2023. We recruited patients to this study who were admitted from home to the heart ward at Akershus University Hospital in Norway and fulfilled our inclusion criteria; they were diagnosed with HF, 65 years or older, managing their own medications, and living in the catchment area of the hospital. We excluded patients who required an interpreter or had a temporarily reduced ability to consent according to the ward nurse. Doctors in this study were either hospital doctors or GPs who attended to patients during the consultations selected for observation. See table 1 for participant characteristics.
Characteristics of participants and audio recorded consultations
We identified and invited eligible patients to participate following these three steps: (1) the project assistant (THBS) screened admission records from the heart ward every morning, Monday to Friday, (2) two researchers (CF and HB) verified inclusion criteria and exclusion criteria with the ward nurse and (3) recruited the attending hospital doctor. We informed all doctors about the study prior to recruiting patients. We observed and audio recorded the following three patient–doctor consultations: (1) first heart ward visit in hospital, (2) discharge visit from hospital and (3) first follow-up visit with GP. Table 1 provides details about the audio recorded consultations. Audio recordings were transcribed verbatim, and observation notes were added when relevant for interpretation of the speech (eg, who was present, what happened during periods of silence, objects patients or doctors pointed to or showed each other). In addition, we collected information from medical records to extract HF history, discharge letters and current prescriptions.
We have used the Strengthening the Reporting of Observational Studies in Epidemiology cohort checklist31 to report how the study was planned and conducted.
Data analysis
This study used Microanalysis of Clinical Interaction (MCI)32, which begins openly, directed by the overall purpose of the project (in this case, how doctors respond to patient utterances regarding what they are doing at home with their prescription medication). Focused inductive work involved listening to recorded consultations and noting observations on transcripts. Working iteratively with a subsample of the material, researchers use MCI to derive essential criteria for how to recognise the phenomenon and develop detailed operational definitions (eg, what constitutes a response). Researchers document the analysis in a coding manual, rendering it transparent and reproducible; they then apply the coding to all recordings to build a systematic and comprehensive collection of the phenomenon of interest. According to MCI, once the collection is complete, researchers characterise the phenomena inductively (eg, how various types of responses differ). The procedures used in MCI can shed light on relationships between the phenomenon of interest and relevant variables such as patient characteristics, the setting or features in the interaction.
In the previous study, we had defined and identified patients’ Medication Adherence Disclosures in Clinical Interactions (MADICI)30, that is, patient utterances to their doctor during medical consultations disclosing their medication adherence, recognised by two essential elements: (1) the utterance is about medications prescribed for use at home AND (2) it includes information about patients’ actions, experience or stance regarding medications. Of the 427 MADICI we identified in the 74 audio recorded consultations, we had found that 235 (55%) included information signalling either a potential risk for non-adherence or outright non-adherence.30
In the current study, we used MCI inductively to explore whether and how doctors addressed these 235 problem disclosures, and how patients responded when doctors’ addressing actions were suggestions for adherence support. How we recognised and characterised MADICI is documented with illustrative examples in our MADICI Codebook, which is available in online supplemental file 1.
Supplemental material
We made three initial assumptions in the current study: (1) patients may disclose problems about different topics (eg, experiencing adverse effects and forgetting to take medications) that they may reiterate in the same consultation or in other consultations, (2) different types of problems may trigger different addressing actions from doctors and should be analysed separately (eg, actions doctors take to address how the patient is experiencing adverse side effects would be different than those to address the patient forgetting to take medications) and (3) doctors’ addressing actions during consultations may be communicated to patients verbally or may be evident in their documented actions.
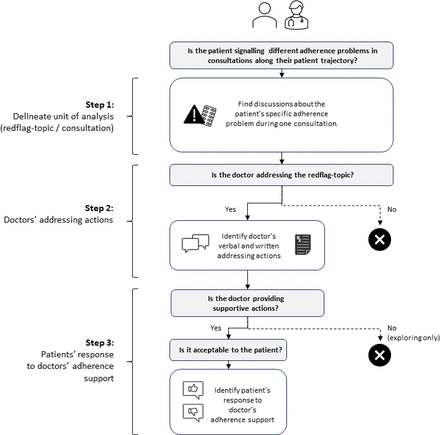
The analysis consisted of three steps (see figure 1). Step 1 was to delineate our unit of analysis, which was any discussion about a patient’s specific adherence problem during one consultation, including anything relevant in the doctor’s written documents about that patient’s treatment plan. Accordingly, for each patient, we collected the previously identified problem disclosures about the same adherence problem into topics (coined as red flag topic). To exploit the study’s longitudinal design, the patient’s first disclosure about the specific problem in any consultation was the entry point for examining all consultations for discussions on that topic. We categorised red flag topics informed by the ‘Perceptions and Practicalities Approach’ (PAPA) framework.18 The PAPA framework focuses on how patients interact with their agreed on treatment and proposes that patients’ adherence to medications is enhanced or reduced by their ability or motivation (or both) to use their medications as prescribed. Whereas motivation influences patients’ conscious (ie, intentional) decision to use or not use their medications, patients with limited practical resources and capabilities are prone to unintentional non-adherence. For each red flag topic, we considered whether the patient signalled a perceptual/motivational adherence problem that could ultimately lead to intentional non-adherence, or a practical/capability barrier that could ultimately lead to unintentional non-adherence.
Flow chart of analytical decisions.
In step 2, we developed operational definitions of doctors’ communicative actions aimed at addressing the red flag topic, and we noted when these actions included adherence support. Then we used a mixed-effects logistic regression to investigate the potential differences between doctors addressing actions of red flag topics that we categorised as either ‘perceptual’ or ‘practical’ in step 1. In the regression, we used doctors’ addressing action as the outcome variable, perceptual/practical as fixed effect, and consultation setting (first ward visit, discharge visit, GP visit) as random effect. Analyses were performed using R (V. 4.4.2) in Rstudio (V. 2023.06.0).
In step 3, we developed operational definitions to identify what feedback doctors received from patients’ responses to their adherence support, that is, whether patients indicated the adherence support was acceptable. The purpose of this step was to ascertain whether doctors’ supportive actions were tailored to patients’ preferences, which foreshadowed the likelihood of those actions improving patients’ adherence situation in the foreseeable future. In consultations where patients changed their preferences during the interaction, we made our analytical decision based on patients’ final response. The coding manual with illustrative examples is available from the first author on request.
We worked iteratively within each step and completed each step before starting the next. When developing operational definitions, we purposefully selected data from three newly diagnosed patients and three patients with known HF. As the definitions coalesced, we gradually expanded our analysis to the full dataset. Developing the definitions started with one researcher (CF) building a collection of examples demonstrating the phenomena of interest in specific, observable actions by listening to audio recordings and investigating written materials. CF used transcripts in Microsoft Excel for reference and for recording all analytical decisions. CF analysed and coded all interactions, meeting with JG regularly to discuss the collection, resolve difficult cases by consensus and refine definitions. Twice we presented examples and preliminary definitions for peer review to a multidisciplinary team of health communication researchers attending our MCI workshop. In addition, CF held individual meetings with one patient representative and several senior medical doctors (cardiology, acute care, general practice) to discuss the relevance of our analytical approach for clinical practice.
Patient and public involvement
The MAPINFOTRANS project was planned with contributions from a user panel consisting of Ahus patient representatives. One user representative participated in MAPINFORTRANS Advisory Board and was consulted to discuss objectives for this analysis.
Results
For each step of analysis, we present our definitions and examples developed during analysis as well as the quantitative results.
Topics of patients’ disclosures of adherence problems
We identified 62 specific adherence problems (red flag topics) in the 235 patient disclosures, which could refer to risks of unintentional non-adherence (n=23, 37%) or intentional non-adherence (n=39, 63%). Unintentional adherence risks related to patients’ internal or external practical problems, and particularly to: (1) healthcare systems-related barriers, (2) limited ability to organise intake of medications in use and (3) limited ability to recall or recognise medications in use. Intentional adherence risks related to patients’ perceptions included: (1) negative stances, (2) negative experiences and (3) concerns or worries. Of the 62 problem disclosures, 34 (52%) were only mentioned during GP visits, 14 (23%) were mentioned in two of three consultations, and three problems (5%) were mentioned in all three consultations. Table 2 presents definitions, illustrative examples and frequencies of topics of patients’ problem disclosures, categorised into types of adherence barriers and unintentional/intentional adherence risk. Details about all 62 red flag topics are provided in online supplemental file 2.
Topics of patients’ disclosures of adherence problems, grouped by patient-oriented adherence barrier
Patients disclosed up to four different adherence problems to their doctors along their patient trajectory; seven patients disclosed one problem, five patients two problems, eight patients three problems and five patients four problems. Analysing 3 key consultations along 25 patient trajectories, we identified that the 62 specific adherence problems appeared in consultations 82 times (recall that the unit of analysis was any discussion about a patient’s specific adherence problem during one consultation).
Doctors’ actions in response to patients’ problem disclosures
We analysed doctors’ verbal and written communicative actions to address patients’ problem disclosures, just after the disclosure or later in the consultation, that could foreseeably change the patient’s situation. These actions were broadly categorised into ‘addressing’ or ‘not addressing’ the patients’ problem disclosure (red flag topic).
Doctors’ addressing actions
We defined addressing as any communicative action that indicates that the doctor is orienting to the patient disclosure by: (1) Exploring the scope of the problem (eg, seeking more information about the patient’s perception or adherence behaviour) AND/OR (2) Providing supportive actions to improve the patient’s ability or motivation to adhere (eg, providing information, prompting, suggesting alternatives to manage the situation, co-reasoning about options, deciding to change prescriptions, ordering professional services).
We observed that the timing of doctors’ responses to patients’ problem disclosures varied greatly. Sometimes doctors would respond immediately, while other times they waited until the patient repeated it. Sometimes doctors delayed their full responses, reintroducing the topic later to discuss how to handle it. We observed some cases where the doctor simply changed the patient’s prescription in response to the patient’s disclosure without discussing it.
As an illustrative example, table 3 presents an excerpt from an interaction where the patient discloses an adherence problem to the GP, who addressed it. In this example, the patient reports forgetting to take medications (line t50-F-4), thereby signalling to the doctor an ongoing adherence problem. After an immediate response to clarify that ‘them’ refers to ‘medications’, the doctor proceeds to address the disclosure by (1) seeking more information about the scope of the problem (line t50-F-7) and (2) providing several types of supportive actions. These include ordering professional services, using alarms and daily routines to reduce the risk of forgetting (lines t50-F-9, t50-F-15), co-reasoning about these alternatives (lines t50-F-19, t50-F-21) and suggesting at the end of the consultation to ‘wait and see’ (line t50-F-23). The doctor provided no additional adherence support to the patient in writing. These addressing actions revealed the scope of the patient’s non-adherence behaviour and provided the patient (and companion) with information that there are many options available to them to improve the situation. The original transcript in Norwegian with translation to English is provided in online supplemental file 3.
Illustrative example of an addressed disclosure
We defined that patients’ problem disclosures remained unaddressed when doctors’ actions were limited to utterances orienting away from the adherence problem by: (1) neutral, non-committal responses (eg, listening responses, reformulating to clarify), (2) pursuing biomedical issues (eg, symptoms, diagnostic tests), (3) changing the topic and (4) emotional and cognitive alignment. In the illustrative example below, from the first ward visit in hospital, the patient discloses how the effect of bumetanide limits his daily activities. This disclosure signals that the patient may have a low motivation to use this medication as prescribed. Here, the doctor immediately provides emotional support (‘no that is a bit of a nuisance’) before pursuing a biomedical issue about the medication (‘Which colour is your urine, is it light or dark’):
Doctor: But what is it like at home?
Patient: Yes it is… straight after I have taken those pills [bumetanide prescribed for use at home] then I have to go to the toilet the next 3–4 hours. But it does not come … it is not a lot though. But I must go to the toilet, I cannot plan any activities as such.
Doctor: No that is a bit of a nuisance.
Patient: Yes, it is. But that’s how it is.
Doctor: Which colour is your urine, is it light or dark?
The patient brought up the same problem during the discharge visit when another doctor presented him with an updated medication list, still including bumetanide. Again, the doctor did not address it. Full transcript with coding notes for both consultations are available in online supplemental file 4.
Frequencies of doctors’ addressing actions
Table 4 presents whether and how doctors addressed patients’ problem disclosures in 82 consultations, organised by topic and consultation setting.
Frequency of doctors’ addressing actions and patients’ feedback
We identified 31 consultations during which patients disclosed problems associated with an unintentional non-adherence risk (ie, patients’ practical problems). In 28 of these 31 consultations (90%), doctors addressed the patient’s problem disclosure either by exploring it further (21 of 28 consultations), providing supportive actions (27 of 28 consultations), or a combination of both. The proportion of doctors who addressed patients’ disclosures of practical problems was high in all settings.
We identified 51 consultations during which patients disclosed problems associated with an intentional non-adherence risk (ie, patients’ negative perceptions). In 37 of these consultations (73%), doctors addressed the patient’s problem disclosure either by exploring it further (23 of 37 consultations), providing supportive actions (36 of 37 consultations) or a combination of both. We observed differences between settings: Doctors addressed patients’ negative perceptions disclosed during the first ward visits 3 of 8 times, 7 of 11 times during discharge visits and 27 of 32 times during GP visits.
We observed differences in how often doctors addressed patients’ problem disclosures indicating different topics and investigated these further. Using a mixed effects logistic regression to estimate potential differences in doctors addressing patients’ disclosures signalling practical or perceptive adherence barriers, we calculated the OR to be 4.79, with a 95% CI 1.25 to 25.83. This result indicates that it is nearly five times higher odds for doctors to address patients’ practical adherence problems (eg, reduced ability to organise intake) to their perceptual problems (eg, negative experiences).
Patients’ responses to doctors’ supportive actions
We observed that patients’ reactions to doctors’ supportive actions varied greatly. While there were some clear indications of acceptance and some outright rejections, sometimes patients would indicate that they preferred another solution, for example, by co-reasoning with the doctor about alternatives or bringing forward ideas of their own. Sometimes there was just silence, which could either indicate that the patient responded only with visible action or did not respond at all.
Based on our observations, we decided to identify patient utterances signalling clear unacceptability to doctors’ adherence support. Our rationale was twofold: (1) working with audio recordings we were missing co-speech gestures and facial expressions, thereby making it difficult to interpret patients’ minimal verbal responses (eg, ‘mm’, ‘yes’, ‘no’) and (2) communication-based research has shown that there is a ‘normative obligation’ for patients to express agreement27 rather than disagreement to doctors’ suggestions, thereby making non-acceptability a more precise indicator for how well doctors’ actions met patients’ preferences.
Patient acceptability
We defined unacceptability as patient utterances that included information that the doctor’s supportive action was against their own preferences or indicated that it was unlikely to change their situation in the foreseeable future. We recognised patient unacceptability when (1) the patient response indicated prior knowledge (eg, information given did not fill a knowledge gap), (2) the patient did not seem convinced by the provided information (eg, gave counter arguments, alternative hypotheses), (3) the patient suggested other supportive measures for the doctor’s consideration (eg, dose reduction, deprescribing), (4) the patient preferred to maintain the status quo (eg, wait and see), (5) the patient did not reject the supportive action outright but shared information that indicated a negative stance or negative experience (eg, told a history of a past experience that did not work) or (6) when the doctor’s prompts were ineffective in revealing reliable information from the patient about their medication use.
Table 5 provides illustrative examples of how we recognised patient’s signals of unacceptability to doctors’ supportive action. The table presents problems that were addressed by doctors, with examples of doctors’ supportive actions (not exhaustive) that the disclosures elicited. Original quotes in Norwegian with translation to English are provided in online supplemental file 5.
Patients signals of unacceptability to doctor’s supportive action
Frequency of patients’ signals of unacceptability
Table 4 presents patients’ feedback in response to their doctors’ suggested adherence support. Nearly 40% of patients responded with negative feedback to their doctors’ suggestions of adherence support. Most problems were discussed during the GP visit, and our results indicate that GPs’ supportive measures were more acceptable to patients than those suggested by hospital doctors.
Patients disclosed topics about healthcare-related adherence barriers only to their GPs, whose supportive actions were always acceptable to patients.
Adherence problems repeated along patient trajectories
So far, all results have been based on single consultations, without taking the longitudinal design into account. Now we will present results for the patients who disclosed the same adherence problem in more than one consultation as they transitioned from hospital to home.
Nearly 50% of HF patients disclosed the same (potential) problem to their attending doctor in different settings. Most of these (n=10) had known HF. They contributed 17 topics in total, about these non-adherence risks: negative experience with medications (n=8), negative stance to medications (n=3), limited ability to recall or recognise medications in use (n=3) and limited ability to organise intake of medications (n=3). Two patients disclosed the same problem in all three consultations. Table 4 also presents a subanalysis of the topics these 12 patients discussed in consultations.
Ten of the 12 patients disclosed a perceptual problem, thereby indicating an intentional non-adherence risk. For two of these patients, none of their doctors addressed the problem. Of the remaining eight, four patients experienced that all doctors addressed their disclosures, and they accepted the doctors’ supportive actions discussed in the GP visit.
Six of the 12 patients disclosed a practical problem, thereby indicating risks of unintentional non-adherence. Doctors always addressed these patients’ problem disclosures. Patients who received help to recall which medications they were using always accepted their doctors’ supportive actions (usually prompts about names and doses). In contrast, patients who struggled with keeping overview and organising their medications never accepted suggestions provided at the GP visit after returning home from the hospital.
Discussion
This is the first explorative study to investigate how doctors and self-managing, older patients with HF discuss patients’ disclosures of medication adherence problems with each other, and how such discussions evolve over time and experience and as patients talk to different doctors. This study offers an ‘inside view’ of how doctors use their communication skills to address patients’ potential or ongoing medication adherence problems, and how in turn, patients respond to their supportive actions. Given the persistently low medication adherence rates in this patient population, a better understanding of this information exchange in practice is valuable to inform practitioners, educators and researchers who work to improve adherence to HF treatment.
The findings showed that nearly 50% of HF patients disclosed the same (potential) problem to their attending doctor in different settings, suggesting that it was an ongoing or recurring issue. Nearly all of them reported problems associated with intentional non-adherence (perceptual issues), while 50% of them reported problems associated with unintentional non-adherence (practical issues). These findings are somewhat surprising given the fact that unintentional non-adherence is considered more common.17 33 One explanation is that due to our recruitment process, patients were more self-efficacious than average HF patients, thereby having the ability to manage their medications well. Another possible explanation for this finding might be patients under-reporting problems since they may prefer to withhold information about their intentional ‘medical misdeeds’.25 34 We observed that doctors’ questions were mainly focused on reconciliation of which medications the patient had been prescribed by other doctors, often failing to follow-up with questions about how patients were managing to use them at home (see table 3 for a good example of eliciting the latter). This observation may be due to time constraints or unawareness of the distinction between the two, but it can also be due to insufficient training in how to elicit information about patients’ adherence behaviour. Health communication research recommends doctors to ‘ask-tell-ask’,15 using open, non-judgemental questions about patients ability to manage their medication intake,35–37 adding explicit questions for precise information about omitted doses.38 This approach also gives doctors the possibility to discover and resolve patients’ misconceptions.39
A second key finding was that most adherence talks took place at the GP visit. Possible explanations for this observation include: (1) junior hospital doctors may prefer to defer challenging discussions (eg, emotional and time-consuming talks) to the patients’ GP who has an established relationship with the patient11 40 41, (2) patients may prefer to discuss problems with their long-standing doctors12 30 42 43and (3) before patients can assess their ability and motivation to adhere to their medications and formulate ‘complaints’, they need time to experience what it is like to use them.
A third key finding was that these doctors addressed most of the patients’ disclosures of medication adherence problems, sometimes by exploring the problem further but most often by providing supportive actions. This finding indicates that doctors were sensitive to and acted on such disclosures, which aligns with previous studies reporting that doctors feel responsible for addressing underlying factors for non-adherence.23 38 However, we found that when doctors addressed patients’ disclosures, they were five times more likely to handle problems associated with unintentional non-adherence (eg, signals of forgetting doses, inability to manage complex regimens, prescription errors) than perceptual problems associated with intentional non-adherence (eg, signals of negative beliefs, low motivation to take medications). When asked, non-adherent HF patients who became adherent decided to do so after understanding how poor their prognosis was without medications12, thereby indicating the pivotal role prognostic talk might have on intentional non-adherence. Though prognostic talk was outside the scope of this study, our impression was that doctors avoided prognostic talk, at least in their responses to patient disclosures. They instead emphasised (biomedical) benefits and necessity of using troublesome medications when patients signalled low motivation to use them (See red flag-topic 5, 24 and 16 in table 5 for examples). Previous studies showed that doctors avoid prognostic talk with HF patients when possible11, which is echoed by patients.12–14 44 Another explanation may be that doctors are unsure how to handle situations where patients signal that their preferences conflict with HF guidelines. Accommodating patients’ wishes by deviating from the best documented regimen for prolonging patients’ lives and reduce hospital admissions3 4 is likely to challenge doctors’ professional standards as well as leave them vulnerable to formal complaints.
Finally, we found that one in two medication adherence problems patients disclosed remained unresolved. Often it was as if patients and doctors talked past each other. Problems remained unresolved due to: (1) doctors did not address patients’ adherence problem disclosures or (2) when doctors addressed it, patients signalled that it was against their preferences or unlikely to change their situation. There are many salient reasons for why doctors left patients’ disclosures unaddressed, including missing the (significance of the) information, downplaying adherence talk given the institutional setting45, in addition to those previously mentioned. In this study, we found that nearly 40% of patients indicated that doctors’ supportive actions were unacceptable to them, leaving their risk of non-adherence unchanged (tables 3 and 5 provide illustrative examples). Patients using their agency to negotiate treatment decisions have been studied in other settings27 46 47, indicating similar levels of unacceptability to doctors’ recommendations.48 The conceptual core of ‘medication adherence’ builds on respect for patient autonomy and patients’ agreement to doctors’ recommended treatment plan.36 49 Therefore, doctors need training and support to develop skills to negotiate and tailor treatment recommendations, both of which are difficult to master in practice.50–52 To conclude, we propose three areas to improve adherence talk: (1) Ensure that all doctors have access to patients’ current prescriptions in one national database, so that doctors can spend less time reconciling what is prescribed and more time assessing patients’ ability and motivation to adhere, (2) train doctors in patient-oriented decision-making regarding medications and how to talk to HF patients about their prognosis, and (3) provide doctors with a ‘toolbox’ for how to negotiate and tailor HF treatments to patient preferences.
Strengths and limitations
The main strengths of this study include: (1) Our findings based on authentic consultations, at three selected time points when guidelines recommend doctors reconcile patients’ prescriptions and talk about their medication adherence.19 20 To explore qualitative aspects of adherence talk, a sample of 74 audio recorded consultations and medical records from 25 patient trajectories have high information power.53 (2) Access to patients’ medical records allowed us to discover doctors’ written adherence support not evident from the dialogue. (3) Our coding manual, available on request, is transparent and reproducible,54 allowing others to apply it in other contexts, ultimately discovering which patterns are unique and which are more universal.
Main limitations of this study include: (1) We recruited patients from one hospital ward, limiting generalisability. However, quantification and comparisons were not intended to support any universal claims; they simply represent the distribution and patterns in the material analysed. (2) All percentages in this study must be considered with caution, given that our sample of 25 patients is not a representative sample of the Norwegian HF population. Due to our inclusion/exclusion criteria and recruitment process, patients may have been less frail than the average HF patient on the heart ward (MAPINFOTRANS included an extended home interview, and several eligible patients indicated they felt too poorly to receive visitors when declining study participation). However, the sample is relatively close in some descriptive statistics to the recent ESC position paper55 and a Norwegian nationwide study.8 (3) The study situation, especially due to an observer recording the consultation, may have led to more talk about medications and ‘best practice behaviour’ from patient and doctor.56 (4) The doctor’s supportive actions were not vetted by other clinicians for their appropriateness in the given situation.
Conclusions
This exploratory study set out to investigate how doctors respond to patients’ medication disclosures indicating a potential or ongoing adherence problem, and in turn, how patients respond to the doctors’ supportive actions that their disclosures elicited. We found that the doctors were more likely to address patients’ adherence problems associated with unintentional non-adherence risks than those associated with intentional non-adherence risks. Even when doctors attempted to address HF patients’ medication adherence problems, half of the problems remained unresolved, usually because patients indicated that the doctor’s suggestions were against their preference.
Data availability statement
No data are available. This study uses audio recorded authentic medical consultations. We do not have permission to share these with other researchers.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants but following review of the project description, the Regional committee for medical and health research ethics concluded that MAPINFOTRANS was exempt from review (ref. 273688). Data used in this study have been collected, handled and stored according to the procedures approved by the Data Protection Officer at Akershus University Hospital (ref 2021_146). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We extend our gratitude to patients and doctors who participated in this study for their time and contributions, and to hospital staff on the Ahus heart ward for facilitating the study. Also, warm thanks to Ivar Bakke for transcriptions and MAPINFOTRANS Advisory Board members and colleagues at HØKH for advice and support.
References
Footnotes
X @palgulbrandsen
Correction notice This article has been corrected since it was published. Reference 32 has been corrected.
Contributors PG, HS, JG and JM conceptualised the MAPINFOTRANS study and applied for funding and ethics approval. HB, CF and THBS conducted the data collection. CF and JG conceptualised the present study, analysed the data and developed the coding manual. TW performed all statistical analyses. CF drafted the manuscript with major contributions to the writing, review and editing from JG, PG, TW and JM. All authors have read and approved the final manuscript submitted for publication. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. The guarantor (CF) affirms that this manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Funding The study was funded by the Norwegian Research Council (291946, 31 August 2021).
Disclaimer The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication. See methods for further details.
Competing interests All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: No support from any organisation for the submitted work; HS has received lecture fees from Amgen, Astra Zeneca, Novartis, Novo-Nordisk and Boehringer Ingelheim; PG has received lecture fees from Norwegian Brain Tumor Society, Pfizer and Takeda; JM is a member of Advisory Committee and Board of Trustees for the International Association for Communication in Healthcare EACH (unpaid) and received lecture fees from Oslo Metropolitan University and EACH; no other relationships or activities that could appear to have influenced the submitted work. All other authors have no competing interest to declare
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.