Article Text
Abstract
Introduction Patients with mental disorders and a history of childhood trauma show an early onset of psychopathology and often a poor response to standard disorder-specific treatments. They represent a patient group which requires more personalised interventions targeting the transdiagnostic mechanisms related to early trauma and its functional consequences. The mechanism-based modular psychotherapy (MeMoPsy) approach is conceptualised as an innovative framework for psychotherapy development. It comprises independent, flexibly applicable interventions from various theoretical backgrounds and evidence-based programmes within a systematic treatment algorithm, thereby tailoring module selection to the specific needs of traumatised adolescents.
Methods and analysis In a randomised controlled feasibility trial (RCT), N=80 outpatients between 15 and 25 years of age diagnosed with various mental disorders will receive 28 individual sessions with MeMoPsy or standard cognitive behavioural therapy. MeMoPsy includes a basic module that addresses trauma history and three additional modules focusing on functional impairments known to be associated with childhood trauma: rejection sensitivity, emotion regulation and relationship difficulties. These modules are selected based on a self-report algorithm. Techniques from mentalisation-based therapy, cognitive behavioural analysis system of psychotherapy, dialectical behaviour therapy and systemic therapy are integrated in this personalised modular procedure. This proof-of-concept study aims to provide initial evidence for acceptability, feasibility and changes in self-rated and diagnostician-rated psychopathology (post-treatment and 3 months follow-up) of MeMoPsy and elucidate the mechanisms of change using psychotherapy process research, Ecological Momentary Assessment and functional magnetic resonance imaging (fMRI).
Ethics and dissemination This RCT obtained approval from independent ethics committees of participating centres and is accompanied by a data and safety monitoring board. Findings will be communicated within the research community as well as with patients and the public by the dissemination strategies of the German Center for Mental Health.
Trial registration number German Clinical Trials Register DRKS00034058.
- Psychosocial Intervention
- Adolescent
- Randomised Controlled Trial
- Adult psychiatry
- Child & adolescent psychiatry
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Psychosocial Intervention
- Adolescent
- Randomised Controlled Trial
- Adult psychiatry
- Child & adolescent psychiatry
STRENGTHS AND LIMITATIONS OF THIS STUDY
This is the first study to investigate the feasibility of a mechanism-based modular psychotherapy (MeMoPsy) for adolescents and young adults with various, frequently comorbid diagnoses and a history of early trauma, thus, a population known to often show poorer treatment responses to standard psychotherapy compared with non-traumatised patients.
Besides feasibility, this randomised controlled trial aims to examine the changes in psychopathology following MeMoPsy to generate pilot data for sample size calculation for a subsequent multicentre confirmatory trial.
Experimental research, Ecological Momentary Assessment, qualitative interviews, as well as regular assessments of the psychotherapy process in patients and therapists will act synergistically to understand the mechanisms of change processes.
Using cognitive behavioural therapy as an active treatment comparator represents a strong comparator for a rigorous evaluation of MeMoPsy with impact for dissemination in mental healthcare services.
Since no a priori values are established, the algorithm cut-offs for module selection used here are based on general population means of self-rated questionnaires (according to a prestudy of our group).43
Introduction
Background and rationale
Childhood trauma experiences such as abuse and neglect are well-established risk factors for mental health problems.1 2 Systematic reviews and meta-analyses consistently indicate robust associations between childhood trauma experiences and a broad range of mental disorders,3 4 such as depression,5 6 anxiety disorders,7 eating disorders,8 substance use disorders,9 psychosis10 and borderline personality disorder.11 Many of those mental disorders first appear before the age of 24,12 making adolescence and young adulthood particularly vulnerable periods.
Since ‘one-size-fits-all’ treatments are not optimal for most patients, innovative approaches focus on more personalised interventions that target the specific functional impairments of patients and associated psychological and neurobiological mechanisms. Patients with mental disorders and childhood trauma experiences are characterised by an earlier onset of psychopathology, more chronic and recurrent symptoms and higher comorbidity rates,13 and—most importantly—they show poorer treatment responses than patients without such trauma experiences.13–17 Thus, the question arises as to why current evidence-based psychotherapeutic treatments appear to be less effective for these patients compared with patients without childhood trauma experiences. One possible reason is that the mechanisms linking childhood trauma experiences to mental disorders are not sufficiently understood and are therefore not adequately addressed in current psychotherapeutic treatments.18 In recent years, numerous mechanisms have been proposed through which childhood trauma experiences could be translated into risk for different mental disorders.19–21 Some of the most prominent transdiagnostic mechanisms underlying childhood trauma experiences and mental disorders encompass (1) rejection hypersensitivity,22 (2) emotion dysregulation,21 as well as (3) difficulties in (close) interpersonal relationships.23
First, individuals with childhood trauma experiences exhibit biases in social information processing, specifically a hypersensitivity towards interpersonal rejection.21 22 Individuals with high levels of rejection sensitivity tend to anxiously expect, readily perceive and over-react to signs of interpersonal rejection.24 According to a recent meta-analysis including 16 studies and 5335 participants, rejection hypersensitivity is linked to childhood trauma experiences, specifically emotional abuse, regardless of age or sex of those affected.22 Moreover, rejection hypersensitivity is associated with specific mental disorders, including depression, anxiety disorders, eating disorders, and borderline personality disorder.22 25
Second, individuals with childhood trauma experiences are characterised by difficulties in emotion and stress regulation.21 Childhood trauma experiences are linked to low emotional awareness, that is, a diminished ability to identify and differentiate one’s own emotions.26 Low emotional awareness may, in turn, contribute to emotion regulation difficulties or emotion dysregulation.21 Emotion dysregulation has been defined as patterns of emotional experiences and/or expressions interfering with appropriate goal-directed behaviours.27 Studies suggest that individuals with childhood trauma experiences are more likely to use maladaptive emotion regulation strategies such as rumination, suppression and impulsive responses.21 28 Likewise, individuals with childhood trauma experiences tend to have more difficulties engaging in adaptive emotion regulation strategies such as acceptance and cognitive reappraisal.21 28 In addition, emotion regulation difficulties emerge in numerous mental disorders, including mood, anxiety, eating, personality and schizophrenia spectrum disorders.29 30
Finally, and closely associated with rejection hypersensitivity and emotion dysregulation, individuals with childhood trauma experiences tend to have more difficulties in (close) interpersonal relationships.23 Specifically, individuals with childhood trauma experiences report more dissatisfaction with current relationships,31 less intimacy,32 33 less social support,34–36 less empathy,37 as well as more loneliness and social isolation38 than individuals without such experiences. Interestingly, difficulties in (close) interpersonal relationships are not only linked to different mental disorders39 but could also mediate the relationship between childhood trauma experiences and mental health symptoms.40
Taken together, a growing body of evidence suggests robust associations between childhood trauma experiences, mental disorders and underlying transdiagnostic mechanisms (ie, rejection hypersensitivity, emotion dysregulation, difficulties in (close) interpersonal relationships). It thus appears promising to target these mechanisms in order to improve current psychotherapeutic treatments for individuals with mental disorders affected by childhood trauma experiences.41
The Center for Psychosocial Medicine at Heidelberg University, together with the Central Institute of Mental Health Mannheim and the Freie Universität Berlin, therefore developed a personalised, mechanism-based modular psychotherapeutic (MeMoPsy) approach for individual outpatient settings. Our MeMoPsy approach builds on a recent proof-of-concept randomised controlled trial (RCT) conducted in collaboration between our research group and Elisabeth Schramm’s research group.42 In this study, 70 adult outpatients between 18 and 65 years with a primary diagnosis of major depressive disorder, at least one comorbid mental disorder and childhood trauma experiences received 20 sessions of either standard cognitive behavioural therapy (CBT) alone or CBT plus modular-based psychotherapy (MoBa). MoBa is based on psychotherapeutic modules, defined as independent but combinable sets of functional units which target common transdiagnostic mechanisms and teach skills to improve processes such as emotion regulation or theory of mind (ToM). In the MoBa approach, three psychotherapeutic modules focus on transdiagnostic childhood trauma-related dysfunctions, specifically social threat hyperresponsivity and social avoidance behaviour, emotion dysregulation, as well as lack of empathy, and ToM. To select these modules in the MoBa condition, a personalised treatment algorithm was applied using empirical cut-off values for self-report measures of childhood trauma-related dysfunctions. First encouraging results indicate the feasibility, safety and efficacy of the MoBa approach, with advantages related to patients’ and therapists’ satisfaction and different clinical outcomes.43
Building on this recent proof-of-concept RCT,43 we aim to assess the feasibility of MeMoPsy in a multicentre, proof-of-concept RCT. We will compare our MeMoPsy approach with standard, non-manualised CBT as CBT represents one of the most prominent treatments as usual in psychotherapeutic healthcare.44 While MoBa targeted adult patients aged up to 65 years with depression, comorbid disorders and childhood trauma, MeMoPsy shifts its focus to the needs of a particularly vulnerable patient group, that is, adolescents and young adults aged 15–25 years with various mental disorders and childhood trauma experiences. Similar to MoBa, the psychotherapeutic modules of MeMoPsy focus on mechanisms underlying the association between childhood trauma experiences and mental disorders (ie, rejection hypersensitivity, emotion dysregulation, difficulties in interpersonal relationships). Further, the personalised treatment algorithm which was used in our previous proof-of-concept study will also be applied in the current study to enable an evidence-based systematic selection of psychotherapeutic modules. We believe that our personalised treatment algorithm represents an advantage compared to the common clinical practice of intuitively selecting psychotherapeutic interventions according to the clinical judgement, expertise and preferences of the treating therapists. Furthermore, the accompanying process research with regular questionnaires on psychopathology and quality of the therapeutic alliance will enable adaptations to patients’ current needs (within the selected modules) depending on patients’ feedback.
Objectives
The aim of this multicentre, proof-of-concept RCT is to investigate the feasibility of a newly developed MeMoPsy approach for adolescents and young adults with various mental disorders and childhood trauma experiences compared with standard non-manualised CBT offered in German mental healthcare services. Specifically, this study aims to (1) examine the acceptability of the MeMoPsy approach for patients and therapists, (2) determine the feasibility of study-related measurements, and (3) investigate the changes in psychopathology following MeMoPsy compared to CBT for sample size calculation of a subsequent confirmatory trial and elucidate the mechanisms of change using psychotherapy process research, Ecological Momentary Assessment (EMA) and functional MRI (fMRI). See tables 1 and 2 for full details of the specific feasibility and other measures used in our study.
Primary outcomes and corresponding measures
Secondary outcomes and corresponding measures
Methods and analysis
The current study protocol adheres to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 2013 Checklist.45 The study protocol’s administrative information relating to the SPIRIT 2013 Checklist and the Checklist itself are presented in online supplemental file 1.
Supplemental material
Trial design
The study is designed as a randomised, controlled, multicentre feasibility trial with two parallel arms (total N=80), comparing MeMoPsy with routine non-manualised CBT. Randomisation will be performed as block randomisation stratified by study site (ie, Heidelberg, Mannheim, Berlin) with a 1:1 allocation.
Study setting
The study will be conducted at three urban German sites, that is, the Center for Psychosocial Medicine at Heidelberg University, the Central Institute of Mental Health Mannheim, and the Institute of Clinical Child & Adolescence Psychology and Psychotherapy of the Freie Universität Berlin.
Eligibility criteria
80 outpatients between 15 and 25 years of age with one or more mental disorders and childhood trauma experiences will be recruited. Patients in the MeMoPsy or CBT condition will be treated by licensed adult and children psychotherapists or psychotherapists in training with at least 2 years of practical experience in treating patients with mental disorders.
Please note that prior psychotherapy experience is not an exclusion criterion; however, prior psychotherapy experience will be assessed in detail in both therapy arms to allow for a comparison regarding familiarity with psychotherapeutic interventions.
The key inclusion and exclusion criteria for patients are as follows:
Inclusion criteria
Age eligibility: 15–25 years
One or more mental disorders according to DSM-5 as assessed with the Diagnostic Short-Interview for Mental Disorders (Mini-DIPS),46 the Structured Clinical Interview for DSM-5 Personality Disorders (SCID-5-PD)47 for avoidant and borderline personality disorders and the Structured Clinical Interview for DSM-5, Clinical Version (SCID-5-CV)48 for attention-deficit hyperactivity disorder (ADHD)
Childhood trauma experiences: at least moderate to severe in one or more of the five subscales of the Childhood Trauma Questionnaire (CTQ; ie, emotional abuse, physical abuse, sexual abuse, emotional neglect, physical neglect),49 as defined by Häuser et al50
Meeting the cut-off of at least one mechanism-based treatment module (modules 1–3)
Statutory health insurance to cover the costs for the psychotherapeutic outpatient treatment
Fluent in German
Written informed consent
Exclusion criteria
Acute risk of suicide, assessed using the Mini-DIPS (interview)46
One or more mental disorders requiring diagnosis-specific treatment as assessed by clinical judgement and applying the Mini-DIPS46 or the 10-item version of the Autism Spectrum Quotient (AQ-10),51 including post-traumatic stress disorder; moderate or severe substance use disorder with the exception of cannabis use disorder; acute psychotic or manic symptoms; autism spectrum disorder
No ability or willingness to abstain from substance use over the course of treatment
Severe cognitive impairment (ie, IQ<70) as assessed with the mini-q52
Other ongoing psychotherapy
Serious medical condition that interferes with regularly attending therapy sessions
Change in current psychotropic medication or initiation of new psychotropic medication for at least 2 weeks before inclusion (3 weeks for fluoxetine)
Interventions
The MeMoPsy condition comprises 28 individual psychotherapy sessions over 24 weeks of treatment (two times per week in weeks 1–4, then once per week in weeks 5–24). Each patient receives a basic module and up to three mechanism-specific therapy modules (figure 1). The application of the modular interventions is preceded by a diagnostic assessment of the patient’s impaired transdiagnostic mechanisms (secondary outcomes, table 2). If the cut-off values of the module-specific questionnaires are exceeded, the respective module will be used for that patient. Building on prior experiences,42 43 53 the module-specific cut-off values are based on adult general population samples. While validation of our empirical cut-off values in an adolescent clinical sample is still pending, all three module-specific questionnaires have been tested in adolescent general populations,54–56 and one of them (ie, Rejection Sensitivity Questionnaire (RSQ))24 has already been proven to be clinically relevant in a previous trial.43 Each module comes with a defined series of interventions, some of which are mandatory, while others are optional for the psychotherapist to use during the course of the therapy. MeMoPsy is a personalised treatment in the sense of an algorithm-driven selection of therapy modules. Furthermore, as feedback on therapeutic processes is an integral part of MeMoPsy,41 a routine outcome monitoring procedure is established, with study therapists receiving access to their patients' questionnaire scores (Brief Symptom Inventory)57 regarding the therapeutic process and psychopathology throughout the treatment.
Modular treatment programme and selection criteria. CBASP, cognitive behavioural analysis system of psychotherapy; DBT, dialectical behaviour therapy; MBT, mentalisation-based psychotherapy; MeMoPsy, mechanism-based modular psychotherapy; OQ-45, IR, Outcome Questionnaire-45, Interpersonal Relations subscale; RSQ, Rejection Sensitivity Questionnaire; S-DERS, State Difficulties in Emotion Regulation Scale.
The therapy modules are as follows:
The basic module, which is mandatory for every patient, encompasses a detailed mental health history, psychoeducation and information about the therapy and the therapy focus, the identification and integration of traumatic experiences, and the improvement of mentalisation and interpersonal functioning. The therapeutic approach is validating, cooperative and influenced by the curious and not-knowing stance from mentalisation-based therapy (MBT).58 Therapists aim to establish a sustainable therapeutic alliance and pay close attention to potential conflicts and ruptures in it. They co-regulate the level of emotional arousal where necessary and identify problematic relationship patterns which may arise as a consequence of trauma. Therefore, therapists use interventions such as the lifeline,59 the window of tolerance model, as well as further techniques from the rupture-repair model60 and MBT.58
Module 1 is administered if patients score ≥9.88 on the RSQ24 (cut-off defined as 1 SD above the general population mean, ie, the upper 16%, as reported in Schramm et al43). It targets interpersonal rejection sensitivity and avoidance behaviour in social situations. It draws on techniques from the cognitive behavioural analysis system of psychotherapy (CBASP)61 such as the significant other history, interpersonal discrimination exercises and situation analyses, and in addition strategies of MBT.
Module 2 is administered if patients score ≥46.97 on the State Difficulties in Emotion Regulation Scale (S-DERS)62 (cut-off defined as 1 SD above the general population mean as reported in Lavender et al62). It aims at improving emotional awareness and stress regulation and draws on techniques from dialectical behaviour therapy (DBT),63 such as emotion-specific psychoeducation, antidissociative or distress tolerance skills, mindfulness exercises and the ability to observe, describe and regulate aversive emotions.
Module 3 is administered if patients score ≥13 on the German version of the Outcome Questionnaire 45 (OQ-45), Interpersonal Relations subscale53 64 (cut-off defined as the 80th percentile of the general population as reported in Lambert et al53). It aims at strengthening resources, resilience and solution focus within the social system of close interpersonal relationships, employing basic principles of systemic trauma therapy65 such as task and goal orientation, resource orientation and solution-focused interventions, but also genograms or relationship maps. This module may take place in an individual therapy setting, but also in multiperson settings with caregivers or other persons of reference if appropriate.
The modules are not simply added as separate and serial components, but therapists will be trained and supervised to integrate them into the dynamic course of the therapeutic process. Consequently, the amount of time spent with a single module will be reduced if more modules are indicated for an individual patient. The therapists are required to use all defined mandatory interventions within the course of a therapy, but beyond that, they will use their clinical judgement and the aid of their supervisors to choose the most effective interventions from the available modules. Therapists will document the time spent with each module and which interventions they use. Altogether, the treatment procedure is algorithm-driven, but allows for a certain degree of flexibility and further personalisation necessary in clinical practice.
The control condition is a treatment-as-usual, non-manualised brief CBT at cooperating psychotherapy training institutes. Patients receive a total of 28 treatment sessions and 3 preparatory meetings, corresponding to the reimbursement scheme of the German statutory health insurance for psychotherapy. Common CBT elements are, for example, psychoeducation, behavioural activation, cognitive restructuring and exposition.
All psychotherapists in both study conditions are supervised by board-certified clinical psychologists or physicians with specialisation in the respective psychotherapy approach, with supervision taking place on average every fourth therapy session, that is, there will be in total seven supervision sessions within a therapy. Psychotherapists in the MeMoPsy condition must complete an intensive training course (four 90 min online theory lessons, 3 days of practical training) held by board-certified clinical psychologists or physicians with specialisation in the respective psychotherapy approach, as well as a pilot therapy of at least 15 sessions with at least 6 additional supervision sessions by the same module experts. In addition, all therapy sessions are recorded on video for the purpose of quality and adherence assurance and can be used as part of supervision.
Outcomes
See table 1 for all primary outcomes and corresponding measures, and table 2 for all secondary outcomes and corresponding measures.
Due to the exploratory nature of this feasibility trial, three primary outcomes were defined: (1) the acceptability of MeMoPsy to patients and therapists, (2) the feasibility of study-related measures and (3) the changes in psychopathology following MeMoPsy compared with standard CBT treatment (see below). Furthermore, a number of secondary outcomes will be explored, including the assumed transdiagnostic mechanisms underlying the link between childhood trauma experiences and mental disorders (ie, rejection sensitivity, emotion dysregulation, difficulties in interpersonal relationships), psychopathological symptoms and psychotherapeutic processes.
Psychotherapy process research will be used to study the course of transdiagnostic mechanisms of change. On a macroscopic level, we will investigate ongoing change, therapeutic relationship and intersession experiences. On a microscopic level, we will address change events, difficult episodes and therapeutic interventions. Findings will be integrated to analyse the action of therapy modules on the course of general psychopathology and well-being as well as rejection sensitivity, emotion regulation and relationship dynamics.
Furthermore, EMA allows us to further operationalise and investigate the assumed mechanisms. First, the dynamics of the mechanisms in everyday life and the connection with the respondents’ well-being can be investigated. Second, potential moderators (eg, personality characteristics) that strengthen or weaken this relationship will be investigated. Third, the intervention effects of the three MeMoPsy modules will be examined experimentally (see below) and in everyday life. Saliva sampling of the stress-dependent hormone cortisol will be linked to ambulatory assessment. All primary and secondary outcomes and corresponding measures are described in tables 1 and 2, respectively. Note that the time points of assessments are given only for the primary outcomes. For a detailed overview of all assessments, see online supplemental table 1.
In addition, neurobiological measurements using fMRI will be performed to elucidate the mechanisms of change initiated by MeMoPsy. Participants complete three tasks in two fMRI testing sessions, one before the start of the treatment and one immediately after the end of treatment. First, the attention bias (ie, increased sensitivity to rejection) is recorded during fMRI using an emotion classification task, in which emotional faces have to be matched.66 Second, the assumed interpersonal difficulties will be measured using an fMRI task on empathy and ToM skills (EmpaToM-Y).67 Empathy and ToM are considered core competencies of social relationship building. The EmpaToM-Y paradigm consists of videos of young actors and actresses reporting specific social settings. This video set enables independent manipulation and assessment of empathy and ToM. Third, the assumed increased sensitivity to rejection and problems in establishing interpersonal relationships are tested using a task on social information processing. This task represents an adaptation and combination of previous studies in which participants rate themselves on character traits and imagine getting feedback for these traits.68 69
Participant timeline
At enrolment, patients will be screened for eligibility, and written informed consent of all eligible patients will be obtained. If the patient is a minor (ie, 15–17 years old), informed consent must also be given by a parent or legal guardian. Consent forms have been adapted to each type of study participant (ie, adult, minor, parent or legal guardian to the participating minor, therapists who participate in the qualitative interviews) at each of the three study sites. For a model consent form for an adult participant at the managing site in Heidelberg, see the online supplemental material. In addition to participation in the intervention study, patients are asked to participate in one or more further assessments using EMA, fMRI, saliva samples to determine cortisol levels, and qualitative interviews. Patients will be randomised to either MeMoPsy or CBT. Data assessments will take place before the beginning of the intervention (baseline, T0), during the intervention (baseline+16 weeks, T1), at the end of the intervention (baseline+32 weeks, T2) and at follow-up (baseline+44 weeks, T3). A comprehensive overview of the frequency and scope of all core trial visits and the continuous outcome monitoring including all assessments and measures is provided in online supplemental table 1.
Sample size
Due to the exploratory nature of this feasibility trial, no formal sample size calculation was performed. Rather, the current feasibility trial serves to obtain pilot data that can be used for the sample size calculation for a subsequent confirmatory trial. For reasons of feasibility, the number of patients in each group (ie, MeMoPsy, CBT) was set at n=40, aiming to recruit four patients per month over a recruitment period of 10 months. With reference to Cocks and Torgerson,70 a total of 80 patients (assuming 20% dropout) is sufficient to obtain data in order to plan a subsequent confirmatory trial for continuous outcome measures for moderate effect sizes of at least Cohen’s d≥0.3. Significant dropout rates of up to 55% have been reported in clinical trials with children, adolescents and adults with childhood trauma experiences.71 In our recent proof-of-concept RCT,43 however, only 5 out of 70 patients (four in MoBa, one in CBT) discontinued treatment prematurely, which corresponds to a dropout rate of 7%. Building on the latter study, the aim of the current trial is to keep the dropout rate below 20%, which is reasonable given that the MeMoPsy approach focuses on the therapeutic alliance and encompasses regular assessments to keep in contact with the patients.
Recruitment
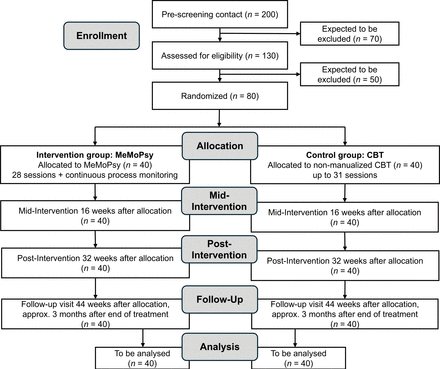
Patients will be recruited at inpatient and outpatient clinics at three German study sites (ie, Heidelberg, Mannheim, Berlin). The majority of patients will be recruited at the Center for Psychosocial Medicine at Heidelberg University Hospital, the Central Institute of Mental Health in Mannheim and the psychotherapeutic outpatient clinic of the Department of Clinical Child and Adolescent Psychology and Psychotherapy at Freie Universität Berlin. In addition, patients will be recruited via posts on social media, flyers in private practices and articles in local newspapers to announce the psychotherapeutic treatment offer within the current feasibility trial. See figure 2 for the trial design and flow of patients.
Trial design and flow of patients. Note that a dropout estimated to amount to 20% may occur along intervention and follow-up. CBT, cognitive behavioural therapy; MeMoPsy, mechanism-based modular psychotherapy.
Assignment of interventions: allocation
Randomisation will be performed, stratified by study site (ie, Heidelberg, Mannheim, Berlin), in a 1:1 allocation ratio. The allocation sequence is based on computer-generated random numbers and implemented using the internet-based software ASMO (Assessment and Monitoring of Mental Health; www.asmo.online), developed and maintained at the Center for Psychotherapy Research at University Hospital Heidelberg.72 The study staff does not have access to the allocation sequence. Patients will be automatically randomised to either MeMoPsy or CBT after having completed the online baseline assessment. The diagnostician accompanying the baseline assessment will inform another member of the study staff after the patient has finished the online baseline assessment. This person will access the result of the randomised allocation sequence provided by ASMO and inform the patient about the allocation to either MeMoPsy or CBT. This procedure enables us to keep the diagnostic staff blinded to treatment allocation.
Assignment of interventions: blinding
Research assistants and diagnosticians involved in recruitment and interview assessments at baseline (T0) and post-intervention (T2) are blinded to treatment allocation. Specifically, blinded diagnosticians will rate the severity of psychopathology using the Global Assessment of Functioning Scale73 at T0 and T2. After baseline assessment, patients receive pseudonymised codes which do not contain any information on treatment allocation. Patients and therapists cannot be blinded regarding treatment allocation due to the nature of the psychotherapeutic interventions. Primary outcomes (except changes in psychopathology following MeMoPsy compared with CBT, see above) and secondary outcomes encompass self-report and therapist-report measures and thus cannot be assessed in a blinded manner. Feedback on psychotherapeutic processes is an integral part of modular psychotherapy.41 Therefore, therapists in the MeMoPsy condition are not blinded to all of their patients’ ratings. Instead, at every fifth session, therapists in the MeMoPsy condition are given access to their patients’ ratings of items measuring changes in the assumed transdiagnostic mechanisms linked to the psychotherapeutic modules, the therapeutic alliance and the psychopathological symptom burden. In addition, at the assessment time points T0, T1 and T2, both the therapists in the MeMoPsy condition and the therapists in the CBT condition are given access to their patients’ ratings of the psychopathological symptom burden. This procedure allows for adapting the selection of interventions according to patients’ current needs (within the selected modules). Research assistants involved
in the additional assessments using EMA, fMRI and saliva samples to determine cortisol levels will be blinded regarding treatment allocation of the patients.
in qualitative interviews are not blinded regarding treatment allocation since they are only performed in the MeMoPsy condition.
in the data analysis will be blinded regarding the treatment allocation with the exception of the data collected using continuous process monitoring which is only done in the MeMoPsy condition.
No circumstances are defined under which unblinding is permissible as both patients and therapists are not blind to treatment allocation.
Data collection, management and analysis
Data collection methods
All patients will participate in comprehensive clinical and experimental assessments, including psychometrically validated, widely used measures (see tables 1 and 2 and online supplemental table 1).
Screening for initial eligibility (time point T−2) will be performed by trained research assistants using a brief screening questionnaire adapted from a prior large cross-sectional study on childhood trauma experiences,74 including the German version of the CTQ.75 Screening will be conducted in a conventional paper-and-pencil format. Diagnostic assessments (time point T−1) will be conducted by qualified diagnosticians (ie, with at least a master’s degree in clinical psychology) who will receive standardised diagnostic training before the beginning of the study. Diagnostic assessments include different commonly used measures captured in a paper-and-pencil format: (1) childhood trauma experiences will be assessed with a comprehensive interview, the KERF-40-I,76 which is the brief German interview version of the Maltreatment and Abuse Chronology of Exposure scale77; (2) mental disorders will be assessed with an efficient interview, the Mini-DIPS46; (3) avoidant and borderline personality disorders will be assessed with the SCID-5-PD47 to consider frequently occurring personality disorders in adolescent traumatised individuals78 79; (4) ADHD will be assessed with the SCID-5-CV48 as it cannot be determined by using the Mini-DIPS; (5) symptoms of autism spectrum disorders will be determined with a self-report questionnaire, the AQ-1051 ; and (6) general cognitive abilities will be measured with a brief screening tool, the mini-q.52 If no exclusion criteria are identified at screening (T−2) and diagnostics (T−1), the software ASMO is used to register the patient and subsequently administer several online questionnaires to measure patients’ and diagnosticians’ ratings at baseline (time point T0). ASMO is used both for the core trial visits (ie, mid-intervention T1, 16 weeks after baseline; post-intervention T2, 32 weeks after baseline; follow-up T3, 44 weeks after baseline) and the continuous process monitoring (ie, patients’ and therapists’ ratings collected every session or every fifth session in the MeMoPsy condition). ASMO allows to automatically collect data on primary and secondary outcomes. The scientific staff ensures that patients and therapists continuously fill out the online questionnaires at the designated time points and, if necessary, reminds patients and therapists by email to complete online questionnaires on time.
All patients will be encouraged to take part in additional optional assessments (EMA, fMRI, saliva sampling to determine cortisol levels and qualitative interviews). All additional assessments will be performed by trained scientific staff under the supervision of experts in the corresponding field.
Once a patient is randomised to one of the two treatment conditions, every reasonable effort will be made to promote patient retention and maximise completeness of data collection. Regular assessments with a maximum interval of 16 weeks will be performed, patients will be reminded to complete the assessments at the designated time points and patients will be financially reimbursed for their participation in the assessments of primary and secondary outcomes, and additional assessments and research tools (ie, EMA, fMRI, hormone measurements, qualitative interviews). Data assessment (T1, T2 and T3) will be administered online, allowing patients to complete it from home and thus reducing their burden related to additional on-site visits. All patients will be asked to participate in the core trial assessments (ie, mid-intervention T1, post-intervention T2, follow-up T3), even if they discontinue treatment prematurely, and thus minimise the number of patients lost to follow-up.
Data management
Data management will be performed using ASMO. The respective servers are located at the University Hospital Heidelberg. Data collected digitally guarantee the highest level of data integrity and quality as risks for missing data and false data entry are minimised. ASMO allows for the monitoring of data collection, the continuous documentation of all access logs, the traceability of all entered data (ie, user and timestamp) and for the restoration of previous states. A Distributed Replicated Block Device-based cluster will ensure synchronous replication of all data during data entry on two separate servers and highest availability. In addition, full and incremental backups will be conducted following a predefined plan. Data storage and data transfer will be encrypted. Access to the data will be password-protected and strictly limited to authorised and trained staff members. Data collected in a paper-and-pencil format (ie, screening for eligibility, diagnostics via interviews at baseline) will be entered electronically by authorised and trained scientific staff using a pseudonymised electronic case report form in ASMO. Data management for the additional assessments will be performed according to standard procedures within the corresponding field.
Statistical methods
Before conducting the final data analysis, a detailed statistical analysis plan will be prepared. Considering the exploratory nature of our feasibility trial, the final data analysis will be performed only descriptively and in accordance with the intention-to-treat principle (ie, based on the full analysis set, including all patients randomised to one of the two treatment groups). All primary and secondary outcomes will be described by treatment arm and overall using appropriate indices from the empirical distributions (ie, arithmetic means, SD, minimum, 25% quantile, median, 75% quantile, maximum, relative and absolute frequencies). For the primary and secondary outcomes, effect sizes between the two treatment groups (ie, MeMoPsy, CBT) will be described in absolute differences and Cohen’s d with corresponding 95% CIs and will be evaluated by unpaired t-tests. For the continuous psychotherapy process research within the intervention group, we follow established standards and employ multilevel modelling to account for the temporal and hierarchical structure of the data. Missing values will be described by relative frequencies and will not be imputed. Patient characteristics between patients with and without missing data in the primary outcomes will be compared in order to identify possible bias. Evaluation of the primary outcomes will be performed blinded to treatment allocation.
For the safety analysis, the frequency of serious adverse events (SAEs) in all randomised patients will be tabulated by treatment group (ie, MeMoPsy, CBT), presumed association with the intervention and severity.
All analyses will be performed in R V.4.4.0 or higher available at https://www.r-project.org/
Monitoring
Data monitoring
A Data Safety Monitoring Board (DSMB) has been established, which is independent of the investigators, the sponsor and of competing interests. The role and reporting structure of the DSMB is detailed in a study-specific DSMB charter, which is available from the corresponding author on request. Briefly, the role of the DSMB is to protect the interests of the trial participants and patients, assess the safety of the interventions during the trial period and monitor the integrity of the trial. In addition, the role of the DSMB is to support and advise the investigators to protect the validity and credibility of the study without violating the underlying study protocol. To this end, the DSMB will meet in person or online on at least three predefined dates (ie, after 25%, 50%, 75% and 100% of the recruitment target has been reached) and as needed (eg, in case of potential safety concerns, delays in study progress). In the DSMB meetings, the recruitment progress, violations of the study protocol, dropout rates, adverse and SAEs, and data quality will be discussed. SAEs, high study dropouts or a high incidence of violations of the study plan may indicate potential safety problems.
The DSMB consists of three German scientists with expertise in psychotherapy research and medical informatics. The DSMB will be supported by individuals with lived experience, who participated in developing the MeMoPsy approach specifically considering the needs of patients (see also the section ‘Patient and public involvement’).
No interim analysis will be performed.
Harms
In the current feasibility trial, SAEs are defined as death of a patient, child endangerment, acute suicidality and acute aggressiveness with indication for inpatient treatment (ie, emergency hospital admission). Adverse events (AEs) are defined as symptom deterioration, occurrence of new symptoms, occurrence of passive suicidal thoughts, problems in the patient-therapist relationship, private problems, occupational problems or other medical conditions. SAEs and AEs are reported by therapists with deterioration of psychopathology also checked by regular session reports. In a comparable, recently published RCT on modular psychotherapy,43 no SAE occurred. In the current feasibility trial, all study-related measures (ie, diagnostics, EMA, fMRI, hormone measurements, qualitative interviews) have already been performed in a similar manner in previous studies by the participating investigators without any SAEs on the study participants. Based on our experiences with psychotherapy trials, we do not expect any SAE to occur in connection with our planned feasibility trial. Should a SAE occur, it must be reported within 24 hours of its occurrence to the principal investigator (SCH), who will forward this information to the members of the DSMB. Indications of SAEs and AEs will be followed up by the diagnostician or the psychotherapist in charge in accordance with clinical guidelines and good clinical practice (eg, consulting with an experienced colleague, initiating child and youth welfare measures, initiating emergency hospital admission). If there are any indications that an adult patient is at risk (eg, recent experience of (serious) abuse or violence), the approach is similar to that for child endangerment, and measures are implemented to ensure safety. Patients with acute suicidal tendencies who require immediate crisis intervention are referred to a suitable specialised facility, but can continue the randomised treatment if the duration of the crisis intervention does not exceed 14 days. Patients who leave treatment due to SAEs will continue to be cared for in accordance with good clinical practice until they are no longer clinically conspicuous.
Auditing
See Data monitoring
In addition, at the request of the study management protocol review, data analysis or similar will be advised. As this is a feasibility study, no external monitoring is planned.
Patient and public involvement
The current feasibility trial is part of the German Center for Mental Health (Deutsches Zentrum für Psychische Gesundheit (DZPG)). The DZPG pursues the overarching goal of promoting population mental health based on a comprehensive translational research programme of national scope (for a concise overview of the DZPG, see ref. 80). Regarding this trial, experts by (lived) experience were involved in the development of the design, in conduct, in reporting and dissemination plans of this research. Experts by (lived) experience have been and will be involved in decision competency in all steps of the research process, which is also reflected by the employment of an expert by (lived) experience in the current feasibility trial who has checked the study design including outcomes.
Ethics and dissemination
Research ethics approval
The study protocol, informed consent forms, recruitment materials and participant information on procedures specific to the hormone measurements have been reviewed and approved by the independent Ethics Committees of the Medical Faculty of Heidelberg University (AZ: S-583/2023), and of the Medical Faculty Mannheim of Heidelberg University (AZ:2023-675). The Ethics Committee of Freie Universität Berlin has declared that it will abide by the vote of the Ethics Committee of the managing site in Heidelberg.
Protocol amendments
All relevant modifications have gained approval by the Ethics Committees in Heidelberg, Mannheim and Berlin prior to the start of the study and have been implemented in the study registration at the German Clinical Trials Register (Deutsches Register Klinischer Studien (DRKS); DRKS00034058). Each study site is responsible for training their study staff in protocol modifications.
Consent or assent
Informed consent will be obtained by qualified psychologists (at least a master’s degree in clinical psychology) trained to ensure adherence to the study protocol. In the informed consent forms approved by the Ethics Committees in Heidelberg, Mannheim and Berlin, patients can consent to the intervention study and the additional measures (ie, EMA, fMRI, hormone measurements), and qualitative interviews separately.
Confidentiality
All data are subject to medical confidentiality and will be handled in accordance with the European Union General Data Protection Regulation (Datenschutzgrundverordnung) and the German legal regulations concerning data protection and security (Landesdatenschutzgesetz Baden-Wurttemberg, Bundesdatenschutzgesetz). All study-related information will be stored securely at the study sites. All data assessed in a paper-and-pencil format will be stored in locked file cabinets in areas with limited access. All data will be pseudonymised (ie, identified by a coded identification number) to maintain patient confidentiality. All data assessed electronically using ASMO will be transferred to the ASMO servers located at Heidelberg University Hospital. Data storage and data transfer will be encrypted. Access to the data will be password-protected and strictly limited to authorised and trained staff members. Data will be stored for 10 years.
Access to data
All investigators will have access to the final trial data set.
Ancillary and post-trial care
Patients can get support from our outpatient and inpatient clinics in case of need.
Dissemination policy
We plan to communicate trial results via publications in peer-reviewed journals and conference contributions. We will use the DZPG newsletter, which addresses people with lived experience (ie, patients and their families), the DZPG website, press releases, LinkedIn and social media for science communication. We will provide access to the full protocol, participant-level data set and statistical code on demand.
Ethics statements
Patient consent for publication
Acknowledgments
We thank Knut Schnell for supervising module 1 and Miriam Biermann for supervising module 2, Corinne Neukel for her advice and support, as well as Ela Gürleyen and Maliwan Müller for their assistance.
References
Footnotes
Correction notice This article has been corrected since it was published. An author name was misspelled.
Contributors KIS, together with SCH, wrote the original draft of the manuscript and developed the basic idea of the study design with support provided by ES, CS and HZ. Details of the clinical trial design were forwarded together by all authors from the three sites. KIS, NS and SF coordinate the study’s implementation and JH the MeMoPsy training and supervision. ST and FE designed and introduced the basic module. ES and SCH designed and introduced module 1; FE, RV and KIS module 2; and MWH and CA-R module 3 of the MeMoPsy intervention. H-CF, together with MW, MWH, NS and SB, drafted the design of the process research; TB and AB-R the design of the qualitative study. MW is responsible for electronic data assessment and management. CWK, supported by KIS and EB, was responsible for designing the fMRI study, BD for hormonal analyses, SvS and LTK for diagnostics and MF for statistical design and analyses. CC is responsible for the study’s implementation at the Berlin study site. EV and JB are responsible for the comparator treatment. All authors contributed to and have approved the final manuscript. SCH is the guarantor.
Funding This study is funded by the Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung (BMBF)) and the Ministry of Baden-Württemberg within the initial phase of the German Center for Mental Health (DZPG) (grant: DZPG 01EE2304B).
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.