Article Text
Abstract
Introduction Increasing awareness of the high frequency, wide spectrum and disabling nature of symptoms that can persist following COVID-19 infection has prompted the investigation of management strategies. Our study aims to determine the effectiveness of atorvastatin on cognitive function, physical activity, mood, health-related quality of life and features of neurovascular impairment and neuroinflammation in adults with ongoing neurological symptoms after COVID-19 infection.
Methods and analysis The STatin TReatment for COVID-19 to Optimise NeuroloGical recovERy study is an ongoing international, investigator-initiated and conducted, multicentre, prospective, randomised, open label, blinded endpoint trial with fixed time points for outcome assessments. A total of 410 participants with long covid neurological symptoms were planned to be randomly assigned to either the intervention group to receive 40 mg atorvastatin for 12 months or to a control group of no treatment, on top of usual care.
Ethics and dissemination This study protocol was designed, implemented and reported, in accordance with the International Conference on Harmonisation guidelines for Good Clinical Practice, the National Health and Medical Research Council of Australia, the National Statement on Ethical Conduct in Human Research and with the ethical principles laid down in the World Medical Association Declaration of Helsinki. Central ethics committee approval was obtained from Sydney Local Health District Royal Prince Alfred Hospital Ethics (No: X21-0113 and 2021/ETH00777 10) in Australia. Site-specific ethics committee approvals were obtained elsewhere before any local study activities. All participants provided written informed consent.
Trial registration number The study protocol is registered at Clinicaltrials.gov (NCT04904536).
- Protocols & guidelines
- COVID-19
- Post-Acute COVID-19 Syndrome
- Clinical Protocols
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study was designed as a prospective, randomised, open label, blinded endpoint trial, comparing atorvastatin 40 mg to standard care for the treatment of neurological symptoms associated with long covid.
The study will be able to systematically and comprehensively characterise patients with neurological long COVID.
The study is open-label, but cross-over to the use of atorvastatin is unlikely, and the primary outcome measure is objective.
Introduction
The epidemiological pattern and clinical spectrum, pathogenesis and complications in people infected with SARS-CoV-2 have been well-described.1 2 However, it is increasingly recognised that many patients have persistent symptoms, in particular, related to cardiorespiratory, mobility, cognitive and psychological function.3–5 This phenomenon has been termed ‘long COVID’ to describe symptoms that continue or develop after acute COVID-19, arbitrarily defined as either ongoing symptomatic COVID-19 (from 4 weeks to 12 weeks) or post-COVID-19 syndrome (12 weeks or more).6
As many as three-quarters of patients have at least one ongoing symptom several months after onset of the infection.7 About 5–10% of individuals experience neurological symptoms, including issues with higher level cognitive functions such as sustained attention, cognitive flexibility and memory, collectively termed ‘brain fog’, along with symptoms such as headaches, dizziness, anxiety, depression, insomnia and fatigue.8–11 Such manifestations may persist even among individuals with mild or absent respiratory symptoms.12 These late neurocognitive problems following COVID-19 could be attributed to: (1) direct non-resolving low-grade inflammation or immune reactions in the brain13 and (2) indirect cerebral injury from hypotension, hypoxia and metabolic dysfunction from effects on the heart, lungs and other organs.4 14 Full neurocognitive recovery post-COVID-19 may take months,11 and akin to other forms of brain injury, it may have long-term impacts on brain health and neurodegenerative conditions such as Alzheimer’s disease.15
Several studies have used specific tests to diagnose neurocognitive symptoms in long covid patients, revealing impairments in executive functions, attention and memory.16 These deficits manifest clinically as difficulties in planning, sustaining focus, recalling information and effectively processing new stimuli, which significantly impact daily functioning and quality of life. Such neurocognitive impairments may be linked to underlying neuropathological changes elucidated through advanced imaging modalities and biomarker analyses.
MRI findings in the brain, particularly of free water quantification using diffusion-weighted sequences, are a sensitive marker of small vessel disease,17 cognitive impairment (especially decision-making performance and working memory)18 and neurodegenerative disease.19 Increases in brain free water reflect an enlargement of extracellular spaces within the cellular matrix of the grey matter or axonal pathways of the white matter due to vasogenic oedema, neuroinflammatory gliosis and/or loss of neuropil or myelin.20 While these changes are traditionally evaluated through histopathological analyses, advanced MRI techniques provide a valuable non-invasive approach to approximating pathological processes.
Additionally, blood markers of endothelial damage, immune function (cytokines/chemokines and acute phase proteins) and neuronal and glial health (eg, neurofilament light chain and glial fibrillar acidic protein) in the acute phase of COVID-19 may function as predictors of long covid.21 These insights underscore the multifactorial nature of neurocognitive symptoms in long covid and the importance of integrating clinical, imaging and biomarker data to better understand and manage this condition.
Statins, widely prescribed for cardiovascular disease prevention, inhibit cholesterol biosynthesis by targeting 3-hydroxy-3-methylglutaryl coenzyme A reductase. They also have anti-inflammatory, antioxidant, vascular endothelial function-enhancing, plaque-stabilising and platelet aggregation-inhibiting effects.22–24 Statins may have neuroprotective effects beyond their cholesterol-lowering properties.25 Epidemiological associations show a reduced risk of all-cause dementia and Alzheimer’s disease in individuals using statins,15 26–29 with effects likely related to type, dose, duration and onset timing over the life course.30 31 Given the key role of the neurovascular unit in modifying the brain’s susceptibility to injury, any benefit of statin therapy is more likely when initiated promptly after an acute inflammatory insult. As atorvastatin crosses the blood-brain barrier due to its high lipophilicity,32 it shows promise as a treatment of neurological symptoms of prolonged COVID-19.33
Here, we outline the protocol for the STatin TReatment for COVID-19 to Optimise NeuroloGical recovERy (STRONGER) study, which aims to determine the efficacy of atorvastatin in primarily mitigating cognitive decline by reducing (or stabilising) neuroinflammation indicated by MRI changes and blood biomarkers.
Objectives
To determine the effects of a standard 40 mg dose of atorvastatin on improving neurological outcomes in adults experiencing persistent cognitive symptoms after acute infection with COVID-19. The primary outcome is cognitive processing speed, assessed by the symbol digit modalities test (SDMT); the key secondary outcome is total white matter free water on MRI. Additionally, the study will investigate other cognitive measures and health assessments and MRI markers, including white matter hyperintensity volume, perivascular space enlargement, grey matter cortical thickness, white matter microstructure, basal ganglia iron load and cerebral perfusion. A cost-effectiveness analysis will also be undertaken.
Methods and analysis
Study design and setting
STRONGER is an international, investigator-initiated and conducted, multicentre, prospective, randomised, open label, blinded endpoint study, with a fixed time point for outcome assessments. Open label indicates that participants and researchers are aware of the treatment allocation. Participants are centrally randomised (1:1) to either the intervention group to receive 40 mg atorvastatin for 12 months on top of usual care or to the control group to receive usual care. Recruitment began on 5 May 2022 and ended in July 2024. The follow-up of participants is until July 2025, and the results are planned to be presented in October 2025.
The study was approved for conduct at three medical clinic sites: the Brain and Mind Centre of the University of Sydney, Monash University in Australia (central ethics approval from Sydney Local Health District Royal Prince Alfred Hospital Ethics, numbers X21-0113 and 2021/ETH00777 10, date of first approval 19 May 2021) and at the Clínica Alemana Universidad del Desarrollo, Santiago, Chile (Comité ético científico: Facultad de Medicina, Clínica Alemana de Santiago, Universidad del Desarrollo, number 2021–75, date of first approval 20 August 2021; government approval: number EC1707587, date: 17 November 2021) as outlined in online supplemental file 1. All subjects provided written informed consent according to the forms shown in online supplemental file 2.
Supplemental material
Supplemental material
Eligibility of study participants
Eligible participants are aged ≥18 years, have a history of COVID-19 that is confirmed by a positive PCR test, a rapid antigen test or as per local guidelines for COVID-19 diagnosis at the time of screening and have ongoing neurological symptoms as a result of COVID-19. These symptoms included problems with memory, concentration, sleep disturbance and fatigue and were systematically identified through administration of the Somatic and Psychological Health Report-34 item questionnaire34 and of any reported loss of smell (anosmia). They must be able to participate in all procedures and provide written informed consent.
Participants were excluded if they had any of the following: evidence of dementia and/or significant cognitive impairment on screening; a severe comorbid medical (eg, renal failure) or psychiatric condition (ie, drug or alcohol dependence and schizophrenia) that prevented participation; history of traumatic brain injury with loss of consciousness (>30 min) within the last 2 years; ongoing long-term use or clear indication or contraindication for statin use; evidence of severe or significant liver disease; creatine kinase levels more than twice the upper limit of normal; being female of childbearing potential and unable or unwilling to use a reliable method of contraception, currently breastfeeding or planned pregnancy. For the subgroup of participants who agree to undergo MRI, they must have had no contraindication due to metallic body parts or claustrophobia. Finally, participants were excluded if their medical history might, in the opinion of their treating physician, put them at significant risk if they were to participate in the trial.
Intervention
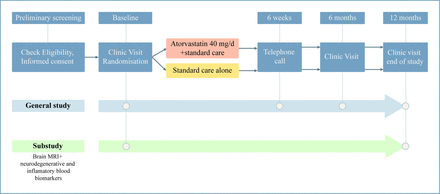
Participants who meet the eligibility criteria were randomised to receive standard care or atorvastatin 40 mg on top of standard care for a period of 12 months (figure 1). The randomisation was stratified by country, time (<6 vs ≥6 months since acute COVID-19 illness), age (<60 vs ≥60 years), current anosmia (yes vs no) that has occurred with temporal relation to COVID-19 and participation in the MRI/biomarker substudy. The randomisation allocation was blinded to researchers conducting the cognitive assessments and endpoint adjudication, and participants, physicians and other study team members were aware of the treatment allocation.
Flow chart of study design.
Visit summary
The study incorporates a blend of inperson visits and telephone interviews (figure 1). Preliminary screening was via telephone or teleconference to obtain informed consent and for review of the inclusion and exclusion criteria. Inperson visits encompass baseline assessments, a clinical evaluation at 6 months and an end-of-study assessment at 12 months. During the baseline visit, various evaluations were performed, including fasting blood tests, clinical assessment, cognitive tests, health questionnaires and physical activity assessments (table 1). Randomisation was undertaken after these assessments were completed, with the participants only being informed of their allocation when all the baseline evaluations were finished, to mitigate the risk of differential withdrawal between the study arms. Furthermore, participants who were enrolled in the substudy are required to undergo MRI scans and blood biomarker analyses at baseline and the end of follow-up. The 6-month visit focused on clinical evaluation and adverse event assessment within the treatment group. On the 12-month follow-up, the baseline assessments are repeated.
Study outcomes
Telephone interviews complement the inperson visits, particularly at the 6-week mark, to evaluate adverse events, medication adherence and changes in concomitant medication. Every effort is made to conduct assessments remotely for participants unable to attend inperson visits.
Outcomes
Primary outcome is processing speed, assessed by the SDMT,35 36 which evaluates frontal lobe executive processing. Participants match numbers to symbols based on a cue of 9 abstract symbols paired with numbers. Performance is measured by the number of correct symbols within 90 s.
Secondary outcomes include a comprehensive battery of assessments covering executive functions, memory, processing speed and other domains, alongside evaluations of health status, MRI and blood biomarkers. A cost-effectiveness analysis, relative to standard care, will be executed. The table provides a detailed overview of all evaluations.
Cognitive assessments are administered by trained research psychologists specialised in cognitive measures. Importantly, these assessments can be conducted either in person or via videoconference.
Safety outcomes
This study documents expected adverse reactions to the study medication of special interest (AESI), including myalgia, nausea, elevated blood glucose, elevated creatine kinase, abdominal pain, new-onset diabetes mellitus and rhabdomyolysis. These AESIs are closely monitored at specific visits to assess participants’ tolerability to study drugs, noting whether they are new or continuing from baseline, regardless of severity. Additionally, all serious adverse events (SAEs)37 and adverse events (AEs) are reported. If treatment is discontinued as a result of any AE, serious or not, the site study team is to provide details of all events that led to the discontinuation of treatment.
Sample size
A sample of 410 patients was estimated to provide 80% power (α=0.05) to detect at least a 0.3 SD effect size difference between groups, assuming equal group participation. These calculations assume a modest 5% non-compliance and 5% dropout over 12 months of follow-up. The SDMT age-adjusted mean score is estimated at 60 (SD 13) at baseline (based on healthy control data).35 The effect is based on trials of the use of statins for the prevention of dementia and trials of treatments for multiple sclerosis, where achieved effect sizes of 0.3–0.4 are clinically meaningful and likely to confer public health benefits.38 For the substudy of participants undergoing MRI, a sample size of 220 (110 per group) is estimated to provide 80% power (α=0.05) to detect an effect size of relative difference of 5.0–6.5 (variance between groups/variance within groups) for the imaging endpoints, assuming a 20% drop out. The study statistician (XW) used the software package PASS V.15 (NCSS, Kaysville, Utah, USA; http://www.ncss.com/software/pass) for these power calculations.
Data collection methods
All data entry is completed via a secure web-based data management system (IBM Clinical Development) at The George Institute for Global Health. Data entry is performed at the participating sites by authorised site staff who have completed training and been given appropriate role-based access to the system. Data logic and consistency checks are programmed into the data entry forms so that data entry errors are captured in real-time and queries are auto-generated. Authorised electronic signatures are used to lock completed data entry forms once all data queries have been resolved within the system. Data entry and all subsequent changes or deletions are captured in an accessible audit trail. Coding of outcomes is centrally performed either automatically via the IBM coding module or manually by the Central Coordinating Centre (CCC). All coding is reviewed and verified by a medical monitor. Reporting within the system is used for regular data reviews and overall trial monitoring. Data are stored and backed up on the IBM cloud servers in the USA.
Statistical analysis plan
The study will follow the intention-to-treat principle for analysis. Baseline characteristics will be summarised by treatment group. Continuous baseline characteristics will be described by means (SD), if approximately normally distributed, or by medians (IQR), if normality cannot be assumed. Categorical baseline characteristics will be presented by frequency per category. Statistical comparisons of baseline characteristics between treatment and control groups are not planned.
The primary endpoint, SDMT score, will be summarised by means (SD) if normally distributed, or by medians (IQR) if normality cannot be assumed. The primary analysis will be conducted using linear regression, with the dependent variable being SDMT and the independent variable being group allocation (treatment vs control) with adjustment for the covariates of age, sex and site. A sensitivity analysis will be undertaken with adjustment for any meaningful baseline imbalances and clinically confounders. Another sensitivity analysis will be conducted by dichotomising SDMT according to population norms (average scores of 54 in Australia and 53.2 in Chile for people aged 44 years) and use of logistic regression models with adjustment for age, sex and study site.
Continuous secondary endpoints will be analysed using linear regression, adjusting for age, sex, study site, unbalanced baseline characteristics and clinically meaningful confounders. Binary secondary endpoints will be analysed using binary logistic regression, adjusting for age, sex, study site, unbalanced baseline characteristics and clinically meaningful confounders. Ordinal secondary endpoints with more than two categories will be analysed using a cumulative logit model, adjusting for age, sex, study site, unbalanced baseline characteristics and clinically meaningful confounders and with testing of the proportional odds assumption.
Descriptive statistics will be provided for safety data, where SAEs and treatment discontinuation will be tabulated using standard terminology. Heterogeneity of the treatment on the primary endpoint will be assessed in the predefined subgroups of age (<60 vs ≥60 years), time since COVID-19 diagnosis (<6 vs ≥6 months), baseline C-reactive protein levels (0–9 vs ≥10 mg/L), ethnicity (white Caucasian vs other) and prior cardiovascular risk (no vs yes for any of a history of hypertension, hyperlipidaemia, current smoker and body mass index (height (cm)/weight (kg) ≥10).2 The hypothesis is that there will be a larger relative treatment effect in younger people, in people with higher levels of inflammation by virtue of an earlier time from the acute COVID-19 infection, in people with raised inflammatory markers, in non-white individuals and those with elevated cardiovascular risk. A detailed outline of the statistical analyses will be specified a priori in a full statistical analysis plan prior to unblinding of the data. A modelled cost-utility analysis using trial data (health-related quality of life, captured by EuroQoL Group 5-Dimension 5-level self-report questionnaire; drug costs; health service utilisation costs, including AEs) will be conducted by comparing use of atorvastatin with standard of care. A 5-year time horizon will be undertaken, with analyses conducted in line with accepted Australian standards for use of economic evaluation in decision-making.
Data monitoring
Trial data are monitored, using central risk-based monitoring principles, to detect any unusual patterns of data that would require further investigation. During the study, representatives of the CCC monitor site performance and quality via remote methods, including via videoconference, to ensure that the study is conducted in accordance with the protocol, International Conference on Harmonisation guidelines for Good Clinical Practice (ICH-GCP) guidelines and relevant ethical and regulatory requirements.
Areas of potential bias
As this is a clinical trial, a major source of bias is selection, so we plan to compare the characteristics of participants with those in published cohorts and between those who were screened. Being an open trial, another main source of bias relates to differential drop-out, for example, participants in the control ‘no treatment’ group failing to return to follow-up assessments or withdrawing from the study. We will try to reduce this by ensuring that all participants fully understand the importance of participation and follow-up, irrespective of ceasing the study medication, and in maintaining good communication and relationships with participants. A final source of bias is behavioural, where participants in either group may change their lifestyle and attitude simply due to participation or in response to factors outside of the trial. We will monitor this by asking questions about use of concomitant medications, diet, smoking and exercise and through use of actigraphy as a proxy measure of sleep and physical activity. There is a low likelihood that the control ‘no treatment’ group will receive atorvastatin (ie, treatment crossover) as this can only be prescribed for people who are at high risk of cardiovascular disease.
Patient and public involvement
The STRONGER study protocol was presented to the Brain Health Consumer Panel of The George Institute for Global Health at meetings in March and November of 2021. This panel comprises a dozen people with lived experience of brain health conditions or their caregivers. Feedback was sought regarding the protocol and patient-facing documents. Those with lived experience provided comments on the feasibility of the study, mode of recruitment, activities and information dissemination. This feedback was incorporated into the study where appropriate, and there was agreement to ensure that the findings of the study, along with a plain language summary, will be disseminated to participants of the trial after the study is published.
Ethics and dissemination
This study protocol was designed and shall be implemented and reported in accordance with the ICH-GCP, the National Health and Medical Research Council, the National Statement on Ethical Conduct in Human Research and with the ethical principles laid down in the World Medical Associations Declaration of Helsinki. Potentially eligible participants are provided with information about the study, and informed consent is obtained from all participants prior to screening assessments.
Writing committees, with oversight by the steering committee, will be formed from members of the various committees, statisticians, research fellows and investigators. Authors of publications must meet the International Committee of Medical Journal Editors39 guidelines for authorship. The study has been approved by relevant ethics committees and regulatory bodies at country level and local sites in Australia and Chile. The current protocol is V.9.0, and all protocol updates have been approved by the Steering Committee and Ethics Committees and communicated with investigators and Data and Safety Monitoring Board members.
Ethics statements
Patient consent for publication
References
Footnotes
X @cheryl_carcel
Contributors CA, SLN, SZ, RP, MW, OH, KL, JE, ML, PM and CC made substantial contributions to the conception and the design of the work. CD, CC, PM and CA wrote the first and following drafts of this manuscript. XL contributed to the statistical analysis and methodology. NW and XS contributed to the critical revision of manuscript for intellectual content. CA is the guarantor of the study. All authors approved submission of the manuscript for publication.
Funding This work is funded by National Health and Medical Research Council/Rare Cancers Rare Diseases, Unmet Need - COVID 19 (MRFF) application ID 2006024, and ANID Fondecyt Regular 1221837 in Chile.
Competing interests CC is supported by an Australian National Health and Medical Research Council (NHMRC) Investigator Grant, Emerging Leadership 1 (APP2009726) and receives research support from Bayer. SN received consultancy fees from Eisai and Roche and speaker fees from Nutrica. MW has recently acted as a consultant to Freeline. RP receives grant funding from the Australian NHMRC paid to her institution. PM receives research grants from ANID Fondecyt Regular 1221837 and a Research Grant from Pfizer 76883481. SZ is employed by Monash University and receives funding from NHMRC, MRFF and the Commonwealth Department of Health and Aged Care. She reports previous payment to institution (Monash University) from Amgen Australia, AstraZeneca, Eli Lilly Australia, Moderna, MSD Australia, Sanofi and Novo Nordisk for participation in advisory and educational meetings. She is a Board Director of the Australian Clinical Trials Alliance and the Victorian Institute of Forensic Medicine. CSA reports research grants from the NHMRC, the Medical Research Council and Medical Research Foundation of the UK, and AstraZeneca. All other authors declare no conflict of interest.
Patient and public involvement Patients and/or the public were involved in the design, conduct, reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.