Article Text
Abstract
Introduction Psychosocial factors impact diabetes outcomes, yet healthcare systems remain inadequately equipped to address these needs. Research centring on the experiences of people with diabetes (PWD) can inform programme implementation, policies and partnerships to address psychosocial care needs. The goals of the diabetes, distress and disparities (3D) study are to quantify the psychosocial care needs of PWD in a large academic medical centre, generate insights regarding how psychosocial factors shape diabetes outcomes and identify modifiable determinants of psychosocial care.
Methods and analysis The 3D study is recruiting adults with type 1 (T1D), type 2 (T2D), latent autoimmune diabetes in adults (LADA) and gestational diabetes (GD) from the Caswell Diabetes Registry at Michigan Medicine. The 3D study uses an explanatory sequential mixed-methods design with two phases. Phase I (P1: target n=500, began July 2023) consists of an online survey to quantify prevalence and examine correlates of a wide range of psychosocial factors (eg, diabetes-related distress, depression, stigma). This survey was refined through consultation with PWD. Phase II (P2) involves semi-structured telephone interviews with n=40 P1 respondents, recruited using maximum variation sampling informed by demographic characteristics and responses to psychosocial survey measures. P2 will explore a subset of factors (eg, patient-provider communication, social support, barriers/promoters to care). To date, n=573 (5% response rate) have completed P1. In March 2024, we identified a target sample of P1 respondents (n=65) for recruitment into P2. All data collection was completed by September 2024. Analysis will involve quantitative linear and logistic regression to understand correlates of psychosocial outcomes from P1, and qualitative content analysis to clarify potential points of intervention from P2.
Ethics and dissemination This study is approved by the University of Michigan Institutional Review Board (HUM00223735). Protocol materials are available at https://osf.io/yfz6b/. Findings from this study will be disseminated through peer-reviewed publications, presentations at conferences and outreach to key stakeholders, including creating educational materials for patient advocacy groups and interprofessional practice.
- general diabetes
- epidemiology
- mental health
- public health
- cross-sectional studies
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The study team worked with people with diabetes (PWD) to refine the survey instrument to help ensure that the content of the survey reflects the issues and experiences that matter most to PWD.
The low response rate to the phase I survey, despite several efforts to augment uptake, required modifications to the overall recruitment goal and analytic plans.
The study interviews only PWD, but the perspectives of their families and healthcare workers are also important to consider in proposing actions to address gaps in psychosocial care.
The study sample is reflective of the patient population seen at Michigan Medicine and reflects the demographics in Washtenaw County, but is not representative of the larger US population.
Introduction
Mental health and diabetes outcomes are linked in a variety of ways that have implications for promoting mental health, diabetes self-management, preventing complications and supporting the overall well-being of people with diabetes (PWD).1 For example, the incidence and prevalence of type 2 diabetes (T2D) is approximately twice as high among people with a history of mental illness (eg, schizophrenia, bipolar disorder, severe depression) compared with those without,2 and certain psychiatric medications further increase the risk of obesity and T2D.3 Mental health also impacts outcomes in type 1 diabetes (T1D). In adolescents with T1D, depression has been associated with increased risk of severe hypoglycaemic and hyperglycaemic events, and depressive symptoms often increase after such events.4 5 While the evidence base is smaller, gestational diabetes (GD) and mental health, including prenatal and postpartum depression, are linked in important ways.6 A history of GD is also a strong risk factor for developing T2D,7 suggesting that understanding the psychosocial experiences of individuals with GD could inform T2D prevention efforts. Finally, up to half of PWD experience diabetes-related distress, the emotional burden and stress specifically associated with managing diabetes,8 which is also associated with poor self-management.9 10 In sum, despite the growing body of evidence indicating a bi-directional relationship between depression and related mental disorders with diabetes clinical outcomes,11 substantial gaps remain in the ability of healthcare systems to meet these psychosocial care needs.12
Building on prior studies of psychosocial needs of people with diabetes
Two landmark international efforts laid the groundwork for our current understanding of the psychosocial care needs of PWD. The International Prevalence and Treatment of Diabetes and Depression (INTERPRET-DD) study (n=2783 adults with T2D aged 18–65 years) examined the prevalence and predictors of mental health conditions among adults with T2D seen in specialty clinics in 14 countries (not including the USA). Overall, this study found that ~11% of adults with T2D had current major depression, yet <30% had been identified by a clinician, with large variability in detection across countries.13 The Diabetes Attitudes, Wishes and Needs (DAWN) studies were two large surveys that sought to quantify psychosocial aspects of diabetes, globally and in the USA, from the perspective of both patients and providers.14 15 DAWN 1 consisted of a sample of PWD (n=5104, including T1D and T2D) and generalist healthcare providers (n=3827 physicians/nurses) drawn from 13 countries (including the USA); this study found that 40% of PWD had poor well-being, yet only 10% had received care for their psychosocial needs.16 DAWN 2 (n=17 countries (including the USA) and n=8596 PWD) was published in 2013 and provided a more detailed assessment of the psychosocial care needs of PWD. The study found that 15% of PWD had probable depression, and 40% had significant diabetes-related distress, yet only 25% said they had been asked by their healthcare team how diabetes impacted their life.15
Collectively, INTERPRET-DD and the DAWN studies demonstrated that psychological distress and depression are common concerns for PWD, both in the USA and globally, and that there are substantial gaps in meeting these mental healthcare needs. Moreover, the American Diabetes Association (ADA) Mental Health Toolkit,17 Standards of Diabetes Care18 and Position Statements by the ADA emphasise the importance of addressing mental, behavioural and social aspects of health as central components of diabetes care.1 However, even with these guiding documents, questions remain regarding the most appropriate setting, frequency and acceptability of these assessments; such guidance is essential to closing the gaps in psychosocial care documented by these landmark studies.1 In addition, the INTERPRET-DD and the DAWN studies spanned multiple types of healthcare and payer systems, but were limited in their ability to identify actionable steps that specific providers or care organisations could take to address the psychosocial care needs identified. Finally, while INTERPRET-DD and DAWN provided a broad understanding of the psychosocial care needs of PWD internationally, they did not fully assess the unique opportunities (and challenges) of the US context. As a result, while these landmark studies demonstrated the scope of the mental health challenges PWD are experiencing around the globe, they could only provide limited insight as to how US healthcare systems should change to address those challenges.
Considering social determinants of diabetes care
Increasingly, healthcare systems are seeking to address, or at the very least assess, social determinants of health (SDOH). The concept of SDOH encompasses factors outside the clinical setting, such as socioeconomic status, neighbourhood characteristics, housing, access to grocery stores and greenspace, food insecurity, social integration and support and experiences of stigma and discrimination.19 Leaders in the field emphasise that policy and programme efforts must address these ‘upstream’ factors to engage in equitable diabetes care.20–22 These social determinants contribute to disparities in healthcare access, and play a profound role in shaping the mental health experiences of individuals managing diabetes. For example, diabetes-related distress is higher among adolescents with lower socioeconomic status,23 and food insecurity is associated with elevated depressive symptoms among adults with T2D.24 A growing body of evidence indicates that addressing SDOH is an essential component of person-centred diabetes care, with positive impacts on both self-management and mental health outcomes for PWD.25 26 However, many questions remain as to how social factors shape mental health outcomes for PWD, and healthcare systems and providers can best address these complex relationships.
Current study
To bridge these gaps in how to address psychosocial aspects of diabetes care, the Caswell Diabetes Institute (CDI) at the University of Michigan recently launched the diabetes, distress and disparities (3D) study of self-management and mental health. This paper describes the rationale, study design, sampling approach, data collection procedures and preliminary findings from the 3D study. The objectives of the 3D study are to: (1) quantify the psychosocial care needs of PWD in a large academic medical centre, (2) generate insights into how psychosocial factors shape diabetes outcomes and (3) identify equitable approaches to address gaps in psychosocial care.
Methods
Study design and eligibility criteria
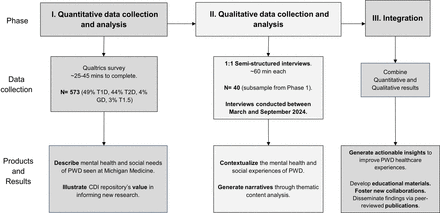
Overview of study design (figure 1). The 3D study uses an explanatory, sequential mixed-methods design, where the quantitative (phase I) and qualitative (phase II) components are directly linked in two ways: (1) the responses to phase I inform the sampling frame for phase II and (2) the responses to the psychosocial measures included in the phase I survey inform the content of the semi-structured interview guide used in phase II. These two phases have distinct but intersecting goals. The quantitative survey addresses a broad range of psychological, social, behavioural and environmental factors and is designed to generate a comprehensive understanding of the breadth of psychosocial care needs of PWD. In contrast, the semi-structured qualitative interviews of phase II are designed to probe select topics in greater depth and to examine processes and relationships that can inform suggestions for improving person-centred diabetes care. Finally, the sequential nature of the design facilitates the integration of qualitative and quantitative information across the phases, through both the purposeful sampling (ie, based on the responses to phase I) and content of the qualitative interview guide.
Study design of the diabetes, distress and disparities (3D) study: an explanatory sequential mixed-methods design. CDI, Caswell Diabetes Institute; GD, gestational diabetes; PWD, people with diabetes; T1D, type 1 diabetes; T2D, type 2 diabetes.
Study eligibility criteria. The 3D study is recruiting adults with T1D, T2D, latent autoimmune diabetes of the adult (LADA, type 1.5) or past or current GD from the patient records at Michigan Medicine. While GD typically resolves after the birth of the child, we included individuals with a history of GD in the sample both because of the established relationship between GD and T2D risk, as well as to address the substantial knowledge gap regarding the potential long-term relationship between GD and mental health.27
Michigan Medicine is a leading academic medical centre that serves a diverse patient population, offering specialised diabetes care and conducting cutting-edge research on diabetes and related conditions. Each year, there are approximately 2.8 million all-cause outpatient visits, 46 000 all-cause hospital discharges and 110 000 all-cause emergency department visits. Persons were eligible if they were aged 18 years or older, had a diabetes diagnosis identified on their problem list and had at least three clinical encounters (ie, office visits, virtual visits, hospital admissions or emergency department visits) in the last 2 years (1 April 2021 through 31 March 2023) by any department at Michigan Medicine. The International Classification of Diseases, 9th Revision (ICD-9) and ICD, 10th Revision (ICD-10) codes used to identify cases of diabetes care: ICD-9: 249 (secondary diabetes mellitus), 250 (diabetes mellitus) and 775.1 (neonatal diabetes mellitus) and ICD-10: E08 (diabetes mellitus due to underlying condition), E09 (drug or chemical-induced diabetes mellitus), E10 (type 1 diabetes mellitus), E11 (type 2 diabetes mellitus), E13 (other specified diabetes), O24 (diabetes mellitus during pregnancy, childbirth and the puerperium) and P70.2 (neonatal diabetes mellitus).
Patient and public involvement
Feedback from PWD and other stakeholders was incorporated into our study design and data collection instruments. First, the team met twice with the CDI Diabetes Patient and Family Advisory Board, including piloting the phase I survey instrument, which resulted in shortening of the length of the survey instrument and expansion of the response options to some questions. Second, due to the lack of racial diversity of the CDI Advisory Board, we conducted two focus groups of PWD through the Michigan Institute for Clinical and Health Research Community Engagement Studio. Third, we consulted with investigators from the INTERPRET-DD and DAWN studies, including reviewing their data collection protocols. While the 3D study is not seeking to be a direct replication of INTERPRET-DD or DAWN, aligning our measurement tools with those of these prior studies reduces the risk of design decisions underlying any disparate findings across these studies. Finally, we consulted with the University of Michigan Department of Family Medicine Qualitative and Mixed-Methods Learning Lab (https://www.mixedmethods.org/) during study design development, which resulted in revisions to the design of phase II from virtual focus groups to semi-structured one-on-one telephone interviews.
Data collection
Fieldwork procedures for phase I
The top half of figure 2 shows phase I participant recruitment in the 3D study. For this phase, potentially eligible individuals are contacted via email by the University of Michigan Data Office for Clinical and Translational Research (DOCTR). DOCTR serves as a broker who securely handles protected health information, which is not shared with the research team unless a person consents to be in the study. The CDI provided DOCTR with ~62 000 adults and their diabetes type data, which was then limited to those with email information (~58 000 individuals). This sample was then split into T1D/LADA, T2D or GD, and emails were sent out in batches of 500: T1D and T1.5/LADA=150, T2D=300 and GD=50. Individuals receive an initial email inviting them to participate (online supplemental appendix A), then two follow-up emails and a final reminder email that their unique survey link is going to expire in 1 month. The subject line of the email was ‘Letter of Invitation to Participate in Research’. The content of the email itself was concise, consisting of two paragraphs that introduced the study, the project coordinator and the principal investigator. To provide additional context, we also included an infographic with detailed information about the study. The data of each email is recorded and tracked, as are intermediate steps (eg, clicking on the email link, even if the person chooses not to participate). As an incentive for completing the survey, participants could provide their contact information to enter into a raffle to win a US$100 gift card; one winner was drawn for every 100 completed surveys. Because participants were anonymous to the research team unless they provided their contact information in the survey, and due to budget constraints, we were not able to provide an incentive to all phase I participants.
Supplemental material
Flow chart of participant recruitment into the diabetes, distress and disparities (3D) study. This flow chart illustrates the participant recruitment process for phases I and II of the 3D study. For phase I, the flow begins with the target sample of patients with diabetes seen at the University of Michigan (UM) and details the steps leading to the final sample of survey respondents (n=573, 5% response rate). Participants in phase II (n=40, 62% response rate) were targeted for recruitment based on their responses to phase I on five features: (1) diabetes control (eg, self-reported haemoglobin A1c), (2) symptoms of depression and anxiety (eg, GAD-7, PHQ-9), (3) symptoms of diabetes distress (eg, PAID-11), (4) history of mental health treatment and (5) social factors such as experiences of discrimination and stigma. We also considered demographic characteristics (eg, age, race, sex, educational attainment and household income) in constructing the phase II target sample. GD, gestational diabetes; GAD-7, Generalised Anxiety Disorder-7; PAID-11, Problem Areas in Diabetes-11; PHQ-9, Patient Health Questionnaire-9; T1D, type 1 diabetes; T2D, type 2 diabetes.
Phase I fieldwork began in July 2023, with an expected response rate of 20% and an initial recruitment goal of 2500 individuals. This sample size was chosen to ensure that correlates of the psychosocial outcomes could be examined both across and within each of the four diabetes types. However, as shown in figure 2, the actual response rate (~5%) is substantially lower than this expectation, although other web surveys have reported similar response rates.28 Therefore, the expected sample for the phase I survey was revised to 500 individuals, with the majority having T1D or T2D (each type has an n~200). However, even with this smaller sample, we will have sufficient power to detect small to moderate effect sizes when comparing across the T1D and T2D groups (eg, we have 80% power to detect Cohen’s d=0.3). We will have sufficient power to detect moderate to large effect sizes within each diabetes group (eg, we have 80% power to detect Cohen’s d=0.4 within the T1D or T2D group). However, the study will no longer have sufficiently large samples of the LADA or GD types to conduct quantitative examinations within these groups.
At the time of the initial submission of this protocol paper (April 2024), fieldwork for both phases I and II was in progress. At the time of the first decision on this manuscript (December 2024), both phases of fieldwork had closed (recruitment for phase I closed in August 2024; recruitment for phase II began in March 2024 and concluded in September 2024). We have updated sample characteristics (eg, table 1) in this paper to reflect the final sample at the time of receiving the first revision request.
Descriptive characteristics of phase I survey respondents and anticipated phase II interview sample of the 3D study
Phase I data collection instrument
The online survey was conducted using Qualtrics, which is Health Insurance Portability and Accountability Act (HIPAA)-compliant. The functionality of Qualtrics means that the survey can be completed using a computer or tablet/cell phone. Items included in the survey were derived from existing health surveys (eg, INTERPRET-DD, DAWN and the Health and Retirement Study Diabetes Study)29 to enhance comparability with these prior studies. The phase I survey takes approximately 40 min to complete and consists of 11 core domains that are asked of all participants, regardless of diabetes type, This is followed by a set of questions relevant to their specific type of diabetes (eg, diabulimia for T1D, weight blame for T2D). The primary psychosocial outcomes assessed by the phase I survey include: mental health (depressive symptoms, anxiety symptoms, diabetes-related distress), stigma/discrimination, behavioural health and social relationships. The primary diabetes clinical and healthcare outcomes assessed in the survey include: glycaemic control, complications, medications (including costs) and technology (eg, continuous glucose monitors, insulin pumps). The primary independent variables and correlates of these outcomes that are assessed in the survey include social and demographic characteristics, healthcare utilisation and diabetes history. Details of the survey content are provided in table 2 and the data collection instrument is available at https://osf.io/yfz6b/. As shown by table 1, 43.5% of phase I respondents have T2D, 48.2% have T1D (this group was oversampled by design), 3.1% have LADA and 4.3% have GD (current or past).
Domains and sources of items measured in the quantitative survey (phase I) of the 3D study
Fieldwork procedures for phase II
The bottom half of figure 2 shows the selection of a subset of participants (~18%) who consented to be contacted for phase II. Selection criteria for interviewees were determined based on the results of the first stage of the study. We used maximum variation sampling (ie, sampling people with either very high or very low values on target constructs)30 in order to capture the breadth of experiences represented in the survey. The five phase I constructs used to inform the sampling of phase II were: (1) diabetes control (indicated by self-reported haemoglobin A1c), (2) symptoms of depression and anxiety (measured using the standardised screening scales, the PHQ-9 and GAD-7, respectively), (3) symptoms of diabetes distress (measured by the PAID-11), (4) history of mental health treatment and (5) social factors (eg, reporting experiences of discrimination and stigma). In addition, we considered diabetes type and demographics (eg, age, race, sex, educational attainment and household income) in constructing the phase II sample to adequately represent the diverse patient population seen at Michigan Medicine.
Eligibility for the second phase of the study is conditional on (1) completing the phase I survey, (2) providing contact information at the completion of the survey (this is the only way that the study team obtains identifiers; up to this point, only DOCTR has contact information for participants) and (3) checking the box at the end of the survey stating that they are willing to be recontacted for phase II. Using the features described above, in March 2024, we selected the recruitment frame for phase II (n=65 phase I respondents, in anticipation of a ~20% refusal rate); the goal sample size for phase II was 50 participants. This sample size was chosen given the multiple psychosocial features used to inform the sampling frame, and the desire for adequate representation across race/ethnicity, age, diabetes type and indicators of socioeconomic status in the qualitative component.
Phase II recruitment involved contacting the n=65 selected participants via email and then telephone to assess interest. Batches of n=10 potential P2 respondents were released every 2–3 weeks to the research staff for recruitment; this staggered approach facilitated problem-solving and team debriefing each week. Once the interview was scheduled, research staff sent a reminder email a few days before the interview to confirm. Interviews, beginning with the verbal informed consent, were audio-recorded using Zoom, which also produced an initial (unvalidated) transcript. All phase II participants received a US$25 gift card to thank them for their time and efforts.
As shown by table 1, 40 individuals completed the phase II interviews (62% response rate); these interviews are currently being transcribed and validated by research assistants (each transcript is validated by two independent raters to ensure accuracy) to prepare them for future analysis.
Phase II data collection instrument
Phase II consists of in-depth semi-structured qualitative interviews conducted by telephone or Zoom. The data collection instrument was created by the study team, which includes a person with T1D and a clinical health psychologist who works with PWD and their families. The goal was to create an approximately hour-long semi-structured interview that addressed psychosocial factors that were (1) prevalent among the sample, based on the phase I survey responses and (2) could provide insight regarding gaps, barriers and promoters to psychosocial health for PWD. The instrument was revised in an iterative process, considering order of questions, stems versus probes and length. The content of the interview is summarised in table 3 and all data collection materials are available at https://osf.io/yfz6b/.
Domains and questions included in the interview guide (phase II) of the 3D study
All staff involved in the phase II fieldwork underwent training in qualitative interviewing by a team member (AR-P) with extensive experience in this method, including role-playing (ie, simulated interviews) and piloting the instrument with multiple members of the study team. Interviewer bias was reduced through the implementation of a standardised interview guide and the systematic training of interviewers.
Linkage with healthcare records
The study team will work with DOCTR to link the survey data for phase I participants to their Michigan Medicine healthcare utilisation records for the calendar years 2023 and 2024. DOCTR will conduct this linkage and then return a dataset to the 3D study team that does not have any personal identifiers, but it will have an anonymous code that will allow us to link these healthcare records to the phase I survey responses. These data will be used to assess metrics of healthcare utilisation, including referrals to social work/behavioural health, emergency department visits, hospitalisations, diabetes-related complications (eg, hypoglycaemic episodes, amputations) and other metrics of care quality.
Data analysis plans
Quantitative data analysis. We will use descriptive statistics (means for continuous variables and proportions for categorical variables) to quantify the prevalence of psychosocial outcomes in the phase I and phase II samples. Initially, this descriptive analysis will focus on four key psychosocial aspects of diabetes: symptoms of depression (PHQ-9), anxiety (GAD-7), diabetes-related distress (PAID-11) and indicators of diabetes-related stigma. As these variables are expected to be right-skewed, we will examine their distributions and apply transformations (eg, mean standardise, median split) as appropriate to better meet the assumptions of the regression models described below.
Table 1 provides an illustration of these types of descriptive statistics: the left column shows the phase I sample (n=573) and the right column shows the phase II sample (n=40). Overall, 8% of phase I participants have elevated anxiety symptoms, 14.5% have elevated depressive symptoms and 23.4% have elevated diabetes-related distress. Next, we will examine whether these psychosocial outcomes vary as a function of diabetes type and clinical characteristics (eg, duration of diabetes, diabetes treatment regimens (oral medications vs insulin)). We will use χ2 tests for categorical variables and analysis of variance, T-tests or Wilcoxon tests (as appropriate, based on the variable distributions) for continuous variables. Finally, we will use linear and logistic regression to examine the association between the four key psychosocial outcomes and glycaemic control (indicated by haemoglobin A1c), accounting for age, sex, socioeconomic status and race/ethnicity. Haemoglobin A1c will be modelled as both a continuous variable (standardised on the sample mean) and as a categorical variable (using clinically relevant thresholds of ≤6.4% for adequate control, ≥8% for poor control). We will examine whether the relationships between psychosocial outcomes and glycaemic control are modified by indicators of SDOH (eg, educational attainment, household income, self-reported financial strain). For all comparisons, the statistical significance will be evaluated using two-tailed tests with a p value threshold of <0.05. Analyses will be conducted using RStudio statistical software.
Qualitative data analysis. We will use the Rigorous and Accelerated Data Reduction (RADaR)31 technique and related approaches for qualitative data analysis to identify prominent themes related to psychosocial aspects of diabetes care. RADaR is an iterative process for analysing qualitative data in a manner that moves between individual and team-based activities. We will use software, such as NVivo, to support qualitative analysis as warranted by the complexity of the research question being addressed. The analytic team will consist of two to four people, who will begin by listening to the audio recordings and reading the transcripts of the semi-structured interviews from phase II, then will create a spreadsheet of all data elements, with text organised by interview questions. This table will then be reduced as appropriate for the specific research question being addressed (eg, analyses examining barriers to care will focus on those probes and responses that address that topic). The team will then begin generating codes individually, which will be refined collectively and applied to the transcripts. They will create a definition and codebook, which will be revised as needed through a process of debate and consensus. The team will then define and apply the (sub)codes and identify illustrative quotes. Given the maximum variation sampling approach used for phase II, the team will examine whether and how themes varied based on the quantitative characteristics (eg, depressive symptoms, glycaemic control) that informed the sampling frame for the qualitative component.
Ethics, data availability and dissemination
The protocol for this study was approved by the University of Michigan Institutional Review Board (HUM00223735) and complies with the HIPAA standards. All participants provided informed consent, whether written (phase I) or oral (phase II). All participants signed a HIPAA waiver as part of phase I that permits researchers to work with DOCTR to link their survey data to their healthcare records (described above). The CDI Repository (REP00000214) was approved by the University of Michigan Medical School Institutional Review Board with a waiver of informed consent and HIPAA authorisation. It uses retrospective data pulled from the Michigan Medicine electronic medical records and does not involve direct interaction with human subjects.
The 3D study was pre-registered in the Open Science Framework (OSF) and all project and data collection materials are available at https://osf.io/yfz6b/. All data are kept on a password-protected and two-factor-protected server housed at the University of Michigan, in a folder that is only accessible by the project team. Only authorised research personnel (eg, principal investigator, project coordinator, select research staff) have access to the identifying information on participants. All team members completed certification through the University of Michigan Programme for the Education and Evaluation of Responsible Research and Scholarship.
Plans for data sharing were described as part of the informed consent process. De-identified data from the phase I survey will be made available through OpenICPSR (https://www.openicpsr.org/openicpsr/) within 6 months of completing data collection. To protect confidentiality, de-identified data from phase I linked healthcare records and the phase II semi-structured interviews (recordings or transcripts) will not be publicly available, but will be available from the corresponding author on reasonable request.
Findings from the 3D study will be disseminated to research audiences at scientific conferences (eg, meetings of the Psychosocial Aspects of Diabetes, American Psychosomatic Society) and peer-reviewed publications. Results will be disseminated to leaders at Michigan Medicine through CDI. Finally, results and their implications will be disseminated to PWD and their families through partnerships with the CDI Patient and Family Advisory Board, advocacy organisations (eg, local chapters of the ADA) and lay media outlets (eg, Psychology Today, The Conversation).
Discussion
It is established that psychosocial factors are a critical component of person-centered healthcare. However, these factors are under-recognized by healthcare providers, and even when they are identified, are often inadequately addressed. Preliminary data from the 3D study demonstrate substantial psychological distress among adults with diabetes, consistent with prior studies. The sequential, explanatory mixed-methods design of the 3D study aims to identify modifiable gaps in addressing psychosocial aspects of diabetes care in a large academic healthcare system. Another strength of our study is the inclusion of diverse types of diabetes (ie, T2D, T1D, LADA, GD). Particularly, psychosocial factors, such as stigma, may operate differently among individuals with GD, often tied to feelings of shame and guilt stemming from concerns around potential harm to the unborn baby. However, few studies have collected psychosocial measures on diabetes broadly (ie, among people with GD, T1D and T2D), in the same sample and with the same instruments, to assess this empirically. By aligning the data collection instruments with those of prior studies, we add to the potential scientific value of the 3D study.
This study has several limitations. First, it is conducted within a single academic medical centre, limiting generalisability to other settings. Second, the study relies on self-reported data, which is subject to both recall error and reporting bias; however, we plan to link these data to healthcare records, which will allow us to quantify and account for these threats to validity. Third, the response rate for the quantitative survey was low, even compared with online surveys in general,32 which may have introduced selection bias. The 40 min survey length may have contributed to this lower response rate. We will examine the ways in which the survey sample differs from the CDI registry (ie, demographic and diabetes characteristics) to quantify and potentially account for this bias by creating sampling weights. Fourth, while the survey assessed a wide range of experiences of PWD, it did not solicit information from families or healthcare workers, who are also important for addressing psychosocial care gaps. Future studies should seek input from these stakeholders as well. Fifth, while DOCTR employed a systematic approach to sending recruitment emails that was based on their experience with prior studies, we cannot entirely rule out the possibility that emails may have gone to spam folders, resulting in a lower response rate. Alternative recruitment approaches, such as mailed letters, were not employed due to financial constraints of the project. Finally, while the sample reflects the demographics of Michigan Medicine and Washtenaw County, it is not representative of the larger US population.
The 3D study will integrate qualitative interviews, quantitative survey measures and clinical healthcare records to generate a comprehensive understanding of the psychosocial care needs of PWD. This knowledge will be shared with advocates and various stakeholders throughout the healthcare system, with the goal of informing the implementation of programmes, policies and partnerships to improve psychosocial care outcomes for PWD.
Ethics statements
Patient consent for publication
References
Footnotes
X @UM_CSEPH
Contributors BM, the principal investigator of the study, led the conceptualisation, methodology and funding acquisition, supervision and edited every draft of the manuscript. CD, the study coordinator, contributed to the conceptualisation, methodology, investigation, formal analysis and data curation, and also wrote the first draft of the manuscript. ARP, research staff, contributed to methodology, investigation, supervision and data curation and edited the manuscript drafts. AE, KM and CZ, research staff, supported data curation, formal analysis and methodology. BF, ES, AM and PC, research assistants, contributed to data curation, investigation and writing, with BF and PC writing sections of the original draft. RM, a co-investigator, contributed to methodology, supervision and reviewing manuscript drafts. CD and ARP contributed equally to this paper. BM is the guarantor of this work and accepts full responsibility for the overall content, including the conduct of the study, access to the data and the decision to publish.
Funding The 3D study is supported by internal funds from the University of Michigan Caswell Diabetes Institute. BM is supported by NIMH/OBSSR (R25MH136652).
Disclaimer The funders had no role in the writing, editing, analysis or decision to publish this paper.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the 'Methods' section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.