Article Text
Abstract
Introduction During the pandemic, overweight and obese adolescents were at a higher risk of COVID-19 infection. Indonesia’s government has implemented prevention programmes and immunisation; however, the rise in SARS-CoV-2 infections among adolescents is exacerbated by low-quality diet and lifestyle habits. Also, the vaccine programme is not prioritised in this population. To address this, a solution involves providing probiotics and counselling on healthy lifestyle habits to improve diet and immunity. Therefore, we designed a protocol for a randomised controlled trial with a 20-week intervention to investigate the effect of probiotics supplementation and counselling on healthy lifestyle habits, including healthy eating and physical activity, and psychosocial stimulation, on nutritional status and antibody response against SARS-CoV-2 in this group.
Methods and analysis This clinical trial aims to investigate the effects of probiotic supplementation on healthy overweight and obese adolescents. The study will involve 440 adolescents aged 12–17 living in Jakarta, Surabaya or Yogyakarta for at least 6 months and have completed at least two doses of the COVID-19 vaccine. The intervention group will receive daily probiotic supplementation of three strains, including Bifidobacterium animalis subsp. Lactis (BB-12), Lactobacillus acidophilus (LA-5) and Lactobacillus rhamnosus (LGG), at the level of 109–1010 colony-forming units for 20 weeks, while the control group will receive a placebo. Both groups will receive weekly counselling on healthy eating habits, physical activity and psychosocial stimulation. The primary outcomes will be changes in the body mass index for age z-score and IgG specific to SARS-CoV-2 titre concentrations between groups. The secondary outcomes will include changes in secretory IgA specific to SARS-CoV-2 titre concentrations, monoclonal antibodies against SARS-CoV-2 spike protein, gut microbiota diversity and the score of Healthy Eating Index 2015.
Ethics and dissemination The study protocol was approved by the Ethics Committee of the Faculty of Medicine, Universitas Indonesia-Cipto Mangunkusumo Hospital (KET 763/UN2.F1/ETIK/PPM.00.02/2022: 1 August 2022). The study results will be disseminated in open-access international journals, scientific meetings and conferences with stakeholders.
Trial registration number The study has been registered at https://clinicaltrials.gov with identifier number NCT05623007.
- Adolescent
- Obesity
- Overweight
- Probiotic
- SARS-CoV-2 Infection
- Randomized Controlled Trial
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The protocol presents a proposed study design of a double-blind, randomised controlled trial that will provide robust evidence on the effect of intervention and minimise selection bias and other potential biases.
The protocol highlights that the study will evaluate the selected immune response to SARS-CoV-2 by measuring serum IgG, secretory IgA and monoclonal antibody affinity, as well as assessing gut microbiota diversity in subsamples.
The protocol emphasises that the study will employ standardised and pretested questionnaires to ensure accurate and reliable results in evaluating the clinical and nutritional outcomes and identifying and controlling for potential confounders.
Background
Indonesia has reported over 1.2 million COVID-19 cases and 34 316 deaths, making it one of the top 20 countries with the highest number of infections.1 2 Jakarta, a megapolitan city, is one of Indonesia’s provinces with many confirmed COVID-19 cases. In addition, 9.1% of the confirmed cases were children and adolescents aged 6–18 years in 2021.2 The confirmed cases and treatment in the isolation room of COVID-19 patients increased by 1.3% and 1.7%, respectively, in February 2022 among that group.3
Although the prevalence of COVID-19 in adolescents in Indonesia is lower than in adults, the fatality rate is among the highest in the Southeast Asian region (2.3%).4 A factor that may have contributed to the high case fatality rate in adolescents in Indonesia is the background high burden of malnutrition.5 The prevalence of stunting and iron deficiency anaemia among adolescents in Indonesia is high (26.9% and 32.0%, respectively) despite a rapid increase in obesity from 2013 to 2018 of 13.5%.6 As undernutrition and overweight have been observed to be risk factors for other respiratory viral infections, it has been postulated that children and adolescents with stunting, obesity and anaemia might be vulnerable to severe SARS-CoV-2 infection.7 8
Adolescence is associated with well-recognised physical health and wellness changes, motivation and behaviour, socioeconomic processes, neural development, neuroendocrine function and cognition.9 10 Adolescents are vulnerable to potential adverse health, social and environmental exposures, such as a variability in the quality of the diet with low fruit and vegetable intake, smoking and alcohol consumption, disturbance in mental health, family disharmony and economic stress; all recognised to be associated with increased risk of adverse health outcomes.11–13 During a COVID-19 lockdown, changes in adolescent eating habits and lifestyles were observed in high-income and low-middle-income countries,14 15 including Indonesia, potentially adding to the risk of malnutrition in this age group.
Nutrition, infection and immunity have a mutually synergetic interacting relationship.16 Undernourished adolescents are susceptible to infection due to compromised immune systems. Alterations in nutritional status directly affect the metabolism and function of immune cells. Nutrition impacts the metabolism and function of T cells by influencing important regulatory signalling proteins via various cytokines and hormones.17 18 Therefore, undernourished adolescents may face a greater risk of getting infected and dying from COVID-19.
Obese adolescents are also vulnerable to many infections, poor disease outcomes and complications, and lower antibody response to vaccinations.17 Studies that have investigated COVID-19 incidence in overweight and obese individuals are still limited in specific populations. We found a study investigating COVID-19 mortality positively correlated with obesity rates in adult populations, even with changes in age, gender and economic contexts.19 In the UK, a report flags that out of 10 465 critically ill patients with confirmed COVID-19, 73.7% were living with overweight and obesity.20
Obesity is recognised as a risk factor for the development of severe COVID-19. In early January 2021, adolescent hospitalisation rates peaked at 2.1 per 100 000, dropped to 0.6 in mid-March and then increased to 1.3 in April. At least one-third of hospitalised adolescents aged 12–17 years needed intensive care, and 5% required invasive mechanical ventilation.21 Besides, obesity is related to a greater risk for critical illness in adolescents (13–21 years) (Adjusted Risk Ratio 3.09, 95% CI 1.48 to 6.47), but not in children (0–12 years).22 These findings are crucial for patient risk stratification and care. Overweight and obesity also seem to be risk factors for worse outcomes in the younger population (<60 years old), with patients with a body mass index (BMI) between 30 and 34 being twice as likely to be admitted to ICU compared with individuals with a BMI under 30.23
Adolescence is also a sensitive period to microbial change11 or dysbiosis due to the potential for inadequate dietary quality, inadequate sleep, stress and substance use (smoking, drugs and alcohol). In turn, dysbiosis can lead to gut inflammation and decreased nutrient absorption.24 Moreover, it may also alter gut integrity, antibody diversity and affinity, ß-cell production and immune system maturation, making them more susceptible to infection in early adulthood.25–27
Recent evidence suggests that the cross-talk between gut microbiota and lung, known as the gut-lung axis (GLA), influences the immune system and the host’s susceptibility to infection, including SARS-CoV-2.28 29 Healthy gut microbiota produces many immune cells that may control lung infection by SARS-CoV-2 compared with dysbiosis in gut microbiota with fewer immune cells. Moreover, the gut microbiota may trigger an inflammatory reaction cascade in response to SARS-CoV-2, leading to viral infection resolution.30 There is also an interrelationship link between diet, probiotics and gut microbiota with antibody affinity.31 Khatri et al32 showed a large interaction surface and strong affinity between SARS-CoV-2 and human angiotensin-converting enzyme (hACE2), a crucial target protein for the SARS-CoV-2 spike protein that lets the virus bind to host epithelial cells. This finding emphasises that improving antibody response and affinity might reduce the risk of COVID-19 infection. Therefore, we designed a double-blind, randomised controlled trial study protocol to investigate the effect of probiotics and counselling on healthy lifestyle habits on nutritional status and antibody response against SARS-CoV-2 in overweight and obese adolescents during the COVID-19 pandemic.
The rationale of the study
Understanding this situation, Indonesia has implemented the COVID-19 prevention programme (wearing a mask, washing hands and social distancing) and the COVID-19 vaccine to protect the population.33 SARS-CoV-2 infections are growing among adolescents. However, this group’s immunisation programme is not prioritised, making the preventive technique less effective. Since exposure among the population, including adolescents, is linked to serious adverse health effects, effective interventions to improve nutritional outcomes and reduce the risk of COVID-19 infection will provide substantial long-term returns. Certain probiotics may help improve BMI for age z-score (BAZ) among adolescents, potentially leading to positive life outcomes for this population during and after the COVID-19 pandemic. However, such interventions in low- and middle-income countries are lacking worldwide. The meta-analysis showed that prophylaxis with probiotics decreased upper and lower respiratory tract infections, sepsis and ventilator-associated pneumonia by 30–50%.34 This indicates that probiotics may become an alternative to improve nutrition and help improve resistance to viral infections, including SARS-CoV-2.
The primary objectives of our study are to investigate the effect of probiotic supplementation combined with healthy lifestyle counselling among overweight and obese adolescents who have been COVID-19 vaccinated on:
Methods
Study design
This study has two phases, but they are not mutually exclusive. In the first phase, we will conduct a cross-sectional study design, which is part of the screening for eligibility, to analyse the biomedical and non-biomedical determinants of adolescent health. The second phase is an intervention study with a double-blind, randomised, controlled trial design with two parallel groups. During the intervention study, the assessment will consist of the baseline, monitoring, assessment (including midline), and endline. The study location will be in Jakarta, Yogyakarta and Surabaya. In Jakarta, we will prioritise the locations in Central and East Jakarta. In Yogyakarta, we will collect data in Sleman City and Sleman Regency. In Surabaya, we will collect data in East, West, South and Central Surabaya. Data collection will be done at school, that is, junior and senior high schools in the study location, including both public and private schools (the priority is a public school).
Eligibility criteria
Cross-sectional study
Inclusion criteria
The subjects must meet all of the following criteria at the time of the cross-sectional study: being male and female adolescents aged 12–17 years; apparently healthy; living in Jakarta, Surabaya or Yogyakarta city for at least 6 months permanently; having completed at least two dosages of the COVID-19 vaccine; the vaccine must be CoronaVac (Sinovac); parents are willing to sign the informed consent form, and adolescents give informed assent.
Exclusion criteria
The cross-sectional exclusion criteria include the following: having severe malnutrition with or without oedema with a BAZ <-3SD; having a history of chronic and non-communicable diseases, congenital diseases and disabilities; reporting current diagnosis as suspected active tuberculosis (TB) and/or history of allergic disease; having a history of gastrointestinal or malabsorption disorders (such as coeliac disease and inflammatory bowel disease) within the last 3 months or during the study; taking other medications; or having diseases that may influence the immune response, that is, immune deficiencies, immunosuppressant medications, blood transfusion or other blood products.
Intervention study
Inclusion criteria
To be eligible for recruitment, the subjects must satisfy all the following specified criteria during randomisation: male or female aged 12–17 years; apparently healthy; living in Jakarta, Surabaya or Yogyakarta city for at least 6 months permanently; being overweight or obese (BAZ >+1 SD); having completed at least two dosages of the COVID-19 vaccine; the vaccine must be CoronaVac (Sinovac); no less than 6 months post-vaccinated before recruitment; parents willing to sign the informed consent (online supplemental appendix 1) and adolescents giving informed assent (online supplemental appendix 2); and must have active health insurance.
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Exclusion criteria
The trial exclusion criteria will include any of the following conditions: having a history of COVID-19 infection within the last month confirmed by PCR or antigen from healthcare facilities or independent laboratory; having a history of chronic and non-communicable diseases, congenital diseases and disabilities; reporting current diagnosis as suspected active TB (primary lung TB, miliary TB hemoptoe, bone TB, meningitis TB); having a history of gastrointestinal or malabsorption disorder (such as coeliac disease and inflammatory bowel disease) within the last 3 months or during the study; taking antibiotics during the 2 weeks before the start of the study (adolescents will be included after 3 weeks of previous antibiotic intake); taking other medications or having diseases that may influence the immune response, for example, immune deficiencies, immunosuppressant medications, blood transfusion or other blood products; taking insulin and/or anti-dyslipidaemia medication; and being pregnant and/or breastfeeding.
Intervention and duration of the study
The intervention group consists of three strains, including Bifidobacterium animalis subsp. Lactis (BB-12), Lactobacillus acidophilus (LA-5),and Lactobacillus rhamnosus (LGG), at the level of 109–1010 colony-forming units (CFU) for each strain, produced by Chr. Hansen, Denmark. Each study participant will be instructed to consume a capsule of the test product once daily, in the morning or the evening. This will be accompanied by counselling on healthy eating, engaging in physical activity, and psychosocial stimulation for 20 weeks. The healthy living counselling will be delivered face-to-face by a certified nutritionist (bachelor’s degree graduates in nutrition). Each of these counselling sessions has specific content. Healthy eating counselling encompasses information on balanced nutrition for adolescents, essential nutrients, appropriate portion sizes, healthy food choices and good eating habits. It also addresses nutrition-related health issues and ways to prevent them. Additionally, the physical activity section focuses on the risks of sedentary behaviour and how to counteract it with appropriate exercise for adolescents. The psychosocial stimulation component addresses the psychosocial needs of adolescents, brain development, and the roles of caregivers, peers and support groups. The control group of participants will be given a placebo similar to the probiotics supplement and counselling on healthy eating, physical activity and psychosocial stimulation. The counselling will be delivered once a week for 20 weeks.
The study will last 20 weeks because it needs a longer duration until a change in BAZ occurs focusing on overweight and obese adolescents with no underlying diseases. A previous study showed significant improvements in BMI and BAZ after 12 weeks of synbiotic use37 and 16 weeks in overweight and obese adolescents with non-alcoholic fatty liver disease.35
Compliance with the consumption of test products will be monitored weekly. We maintain good communication through daily personal messages with the subjects to avoid non-compliance and dropout. Good compliance is defined as taking supplements 6 days/week. Compliance is assessed based on subjects’ recording of missing doses. Compliance should be at least 80% of the total frequencies for the return supplementation package.
Sample size
The minimum sample size calculation for antibody concentration is 440 subjects for two groups of intervention and control (175 subjects are needed for each arm; after adding 20% dropout, 220 subjects per arm will be recruited). The sample size will enable us to detect a 22% change in the antibody level specific to SARS-CoV-2 based on the study data from Rizzardini et al36 with a pre-set level of significance (probability of type I errors) of 5%, a power (probability of type II errors) of 80%, two-sided testing, and taking 20% potential dropouts and incompliant cases into account.
Randomisation and blinding
Adolescence will be randomised in a double-blind manner to two groups. Randomisation sequences will be created using Microsoft Excel with a 1:1 allocation using random block sizes of 2 and 4, stratified by age, sex and region (Jakarta and non-Jakarta). An independent researcher will do the randomisation. The group allocation information will be kept confidential to the researcher and subjects until data analysis.
Outcome measures
The primary outcome measures in this study are the mean changes in BAZ and changes in IgG specific to SARS-CoV-2 titre concentrations. BAZ will be analysed with WHO AnthroPlus at baseline, monitoring and endline. IgG and IgM titre concentrations against SARS-CoV2 will be assessed by electrochemiluminescence immunoassay at baseline and endline. The secondary outcome measures (Table 1) in this study are α and β-gut microbiota diversity, monoclonal antibody affinity against SARS-CoV-2, sIgA specific to SARS-CoV-2 titre concentrations and dietary quality. Table 2 describes the outcome measure description and time frame.
Variable indicator matrix for primary, secondary and other outcomes
Outcome measures and schedule of intervention and assessments
Besides primary and secondary outcomes, the study also measures other outcomes, including other indicators for nutritional status, COVID-19 infection, physical activity, gut microbiota profiling, gut integrity and inflammatory biomarkers, cognitive function, morbidity and mortality, behaviour/mental disorder and self-esteem and quality of life. The outcome measures for these variables are presented in Table 1.
Study procedure
The school’s study site will be selected using probability proportional to size sampling.38 The selection of schools will be based on these criteria: public schools in urban areas have good cooperation, and school principals and teachers are willing to support the study. Informed consent will be obtained from parents or guardians by sending formal mail with support from school authorities. Field workers supervised by research team members will explain the research aim, goals and activities to adolescents at school. Adolescents will be recruited after their assent. The intervention study will be conducted on subjects who meet inclusion and exclusion criteria, with random assignment by an independent researcher. All subjects and the main investigator remain blind until the results are analysed. Stratified-block randomisation is used to control and balance covariate influence. The study involves participants in two groups: one receiving probiotics and healthy living education for 20 weeks and the other receiving a placebo and healthy living education for 20 weeks.
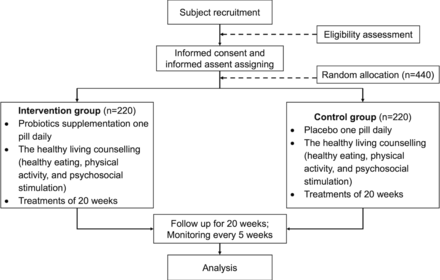
Once an adolescent is enrolled or randomised, the study site team will make every reasonable effort to follow the adolescent for the entire study period. It is projected that the rate of loss-to-follow-up on an annual basis will be at most 5%. Study site staff are responsible for developing and implementing local standard operating procedures to achieve this level of follow-up. The research flow of the study is illustrated in Figure 1.
Flow chart of the Study.
Data collection
Data collection will be conducted at baseline (week 0), during monitoring every 5 weeks (weeks 5, 10 and 15) and at endline (week 20). After endline, we follow-up again at week 24. The data collected are subjects’ sociodemographic characteristics, nutritional status, COVID-19 antibody response, dietary intake, physical activity, gut microbiota profiling, gut integrity and inflammatory biomarkers, cognitive function, morbidity and mortality, behaviour/mental disorder and self-esteem, peers’ influence and quality of life.
Subjects’ sociodemographic characteristics and peers’ influence will only be obtained at baseline. Subjects’ sociodemographic characteristics include age, gender, ethnicity, urban/rural, family determinants (parents’ education, occupation, income, family structure) and monthly pocket money. These data will be collected from subjects through interviews by the researcher using a questionnaire based on Indonesia’s demographic and health survey.39 Meanwhile, peer influence and parenting support will be collected through interviews using a validated questionnaire adapted from Haidar et al40
Nutritional status data will be collected through anthropometric measurement at baseline, during monitoring and endline. Body weight, height, mid-upper arm circumference (MUAC), waist circumference and hip circumference will be measured. Body weight will be measured using the SECA digital scale with an accuracy of 0.1 kg. Body height will be measured using the ShorrBoard stadiometer with an accuracy of 0.1 cm. MUAC, waist circumference and hip circumference will be measured with measuring tape to the accuracy of 0.1 cm. Subjects will be measured based on standard procedure with minimal clothing and accessories. All measurements will be conducted twice to assure accuracy. The final measure will be the mean of the two measurements.
Dietary intake will be taken at baseline, during monitoring and at the endline. Data will be collected using two non-consecutive 24-hour food recalls and frequency questionnaires. A competent nutritionist will take these data. Based on the 24-hour food recalls, dietary quality will be analysed using the Healthy Eating Index 2015.41
Physical activity will be taken at baseline, during monitoring and endline. It will be collected through interviews using the International Physical Activity Questionnaire for Adolescents. Meanwhile, standard questionnaires will also be used to collect data on behaviour/mental disorders, self-esteem, cognitive function and quality of life (Table 1). These variables will be taken at baseline and endline. Morbidity and mortality data will include the history of illness, including COVID-19, gastrointestinal symptoms and medication history.
Blood samples will be collected from subjects at baseline and endline to obtain data on COVID-19 antibody response (total IgG, IgM and neutralising antibody titres), inflammatory biomarkers (hs-CRP), gut integrity (zonulin) and routine blood measurement (haemoglobin, erythrocyte, leucocyte, and thrombocyte count and blood sedimentation rate). 25 mL venous blood for each parameter will be drawn after 12 hours of fasting. All the blood samples will be stored at −80°C until further analysis. Faecal samples will be collected at baseline and endline to assess gut microbiota profile and inflammatory markers. Assessment of gut microbiota profiling will focus on the types of phyla and genera of bacteria and their relative abundance, which includes both α and β diversity. This will be conducted using next-generation sequencing (NGS) of 16S ribosomal RNA (rRNA). Faecal samples will be collected in standard stool collection tubes. The samples will be transported immediately (within 2 hours) at room temperature and stored at −80°C until further analysis.42 Inflammatory markers will be assessed in the stool samples, including faecal calprotectin, as well as faecal zonulin and occludin as a marker of gut permeability.43 44
Adverse events
According to the definitions, adverse events are any untoward medical occurrence that follows the probiotic supplement given to the subjects and does not necessarily have a causal relationship with the probiotic usage. The adverse event may be any unfavourable or unintended sign, abnormal laboratory finding, symptom or disease. The adverse events are also collected from those who withdraw or are lost to follow-up. The safety data will be collected up to 1 month after the last probiotic supplement is given. During this period, each subject will be provided with a diary card to record the appearance, duration and intensity of any systemic event, expected or not. For any subject, a systemic event will be defined as the occurrence of one or several symptom(s) within 28 days following dosing. The systemic events will include nausea, bloating, constipation, thirst, headache, migraine, itching, watery eyes, runny nose, trouble breathing, fever, anxiety, diarrhoea and vomiting.
Adverse events will be collected as indicated in the Diary Cards. Adverse events related to the product, whether serious or not, that persist at the end of the trial will be followed up by the investigator until their complete disappearance. The investigator informed the medical responsible or the monitor of the date of final disappearance of the adverse event and documented it on a case report form. Moreover, any serious adverse event likely related to the product occurred after the investigator reported trial termination to the probiotic’s product pharmacy according to the procedure. A serious adverse event (experience) is any untoward medical occurrence at any dose, including results in death, life-threatening situations, requiring inpatient hospitalisation or prolongation of existing hospitalisation, and results in persistent or significant disability/incapacity. Every serious adverse event occurring throughout the trial will be reported to the Data Safety Monitoring Board (DSMB), the Sponsor and the Ethics Committee, by the investigator as soon as he/she is alerted to it, that is, within 24 hours, even if the investigator considers that the adverse event is not related to the treatment.
Data management
After filling out a paper form, the field enumerator will see the study participant complete a self-administered questionnaire. The field supervisor will oversee the enumerator personnel before, during, and after data collection. After gathering the data, the enumerator will confirm its quality and completeness. Supervisors assess field observation and questionnaire quality. Field data will be centralised and divided by category. The field supervisor and database coordinator will carry out the final check. Data verification involves checking for consistency, missing stages, data completeness and other important information.
A central database will hold all subject data. Database coordinators back up data. All topics are confidential. All data will be entered into digital databases in Indonesian. Information collected in a conservation zone is private. Data are exclusively used for study. Passwords will secure all digital files. The nutrition specialist-led research included face-to-face interviews and self-administered questionnaires. The samples included blood collected by a qualified phlebotomist and stool samples collected by the participants. The principal investigator will validate data entry, and the field supervisor and database coordinator will review the data. Per informed consent, each subject received a unique ID identifier to protect their privacy.
Data will be encrypted and authenticated by authorised users. Access will be limited, monitored and audited. Identity-tracking data will be kept secure, and personal information will be private. Sensitive data will be identified early. Encrypting data on portable devices is stressed as a crucial step in preventing unauthorised access.45
The principal investigator will have unrestricted access to the data sets specific to their particular location. In order to maintain anonymity, all identifiable participant information will be removed from the data before it is shared with project team members.
Statistical analysis
The intention-to-treat analysis will be performed for all eligible adolescents who have received the supplementations at least once and continue to be followed up on for the endline measurement. The complete case analysis will include all participants who completed the endpoint examinations. The per-protocol analysis will consist of the participants who will complete the endpoint examinations, have compliance with the supplement, access and apply the education module, and have no intake of other probiotic products during the study period.
Statistical analysis will be performed using SPSS V.20 for Windows. One sample Kolmogorov-Smirnov test will be used to check the data’s normality. For normally distributed data, the independent t-test (corrected for multiple comparisons) will be used. For non-normally distributed data, the Mann-Whitney U test will be applied. Data will be expressed as mean±SD for normally distributed parameters, while median and 25th–75th percentiles for non-normally distributed parameters will be presented.
The χ2 test will be used to compare group variables (such as sex and age group). Results will be considered statistically significant at p<0.05. The odd ratios (ORs) estimates will be conducted using logistic regression. The hazard ratios (HRs) and relative risks (RRs) for binomial data will be accompanied by 95% Confidence Intervals (CIs). Two-sided significance tests will be used, with p<0.05 considered statistically significant.46
One of the main parts of the analysis will be to compare the changes in mean antibody levels between the endpoint and baseline for intervention and control groups depending on the time elapsed after the full vaccination and history of COVID-19 infection before and during the intervention. For this purpose, the two-way ANOVAs (time factor, two levels; COVID-19 factor, two levels) will be conducted for both groups to compare the mean changes in the antibody level.
In addition, to address the missing data issue, we will analyse the univariate disparities between those who completed the task and those who did not finish, including χ2 and multivariate analysis of variance (MANOVA). Then, we will use logistic regression analysis to investigate the factors that exhibited a significant correlation with an increased likelihood of discontinuing participation in the research at a multivariate level.47
Discussion
Since January 2021, the Indonesian government created the COVID-19 prevention programme (wearing a mask, washing hands and social distancing), strengthened 3T (tracing, testing, treatment) and the COVID-19 vaccine to protect citizens. Based on the current clinical trial, antibody levels with the CoronaVac vaccine started to decline 3 months after the second dose,48 and they reached a low level by 6 months after the second dose.49 50 Preventing SARS-CoV-2 infections in children and adolescents may become less effective because policy often ignores poor diet, lifestyle and home-based family cluster transmission. Therefore, COVID-19 may be particularly prevalent in children and adolescents due to these factors.10 12 13 Effective dietary therapies and COVID-19 risk reduction will have long-term advantages since adolescent exposure causes substantial health concerns.
Currently, there is a suggestion that probiotic intervention can benefit health. These effects include reducing disease symptoms, preventing diarrhoea and respiratory infection,51 52 influencing growth52 and improving the immune system by promoting the colonisation of beneficial gut bacteria. This can impact the gut-lung axis pathway. A meta-analysis suggested that a probiotic resulted in a 30–50% reduction in upper and lower respiratory tract infections, sepsis and ventilator-associated pneumonia.34 This suggests that probiotics might serve as an adjunct to improve nutritional status and reduce susceptibility to viral infections, including SARS-CoV-2 infection.
This study provides an opportunity to assess the effect of a probiotic on mean changes in BAZ and changes in IgG specific to SARS-CoV-2 titre concentrations, α and β-gut microbiota diversity, monoclonal antibody affinity against SARS-CoV-2, sIgA specific to SARS-CoV-2 titre concentrations and dietary quality.
To interpret the results for the changes in total serum IgG against SARS-CoV-2, it is important to consider whether they are influenced by vaccination,53–55 history of COVID-19 infection53 56 or the probiotic intervention.57 If an individual who starts with a low IgG titre level at baseline shows an increase of serum IgG titre by 22% as expected,36 it may be interpreted as them having been exposed to COVID-19 and having mounted an immune response,53 56 as they presumably do not have a more prolonged vaccine effect.53 However, this does not necessarily imply that the probiotic made them susceptible to reinfection.57 On the other hand, no changes in IgG titre should also be interpreted with caution,58 59 as it may indicate either the success in preventing COVID-19 or the lack of effect of the probiotic. To ensure accurate statistical analysis, controlling for confounding factors such as the duration between blood sample collection and the second vaccination dose and any previous or current history of COVID-19 infection is important.
To determine whether probiotics have an effect, we have predefined that a higher increase in total serum IgG levels in the probiotic group compared with the placebo group will indicate their efficacy after controlling for the aforementioned confounding factors.
Some probiotics also improve the diversity and balanced composition of the gut microbiota and affect body weight in obese individuals. Probiotics exert several mechanisms against obesity, including antimicrobial activity, enhancing barrier function and improving the gastrointestinal immune system (immunomodulation).38 Introduction to probiotics during adolescence can alleviate inflammation and reverse dysbiosis.39
This study has some potential strengths and limitations. This study performs a double-blind randomised controlled trial to provide evidence regarding the effect of probiotics combined with counselling on healthy lifestyle habits focused on overweight and obese adolescents during the COVID-19 pandemic. The study design will also reduce potential bias from both researchers and subjects commonly found in community nutrition research. The clinical and nutritional outcomes will be assessed using a standardised questionnaire to detect potential confounding variables, thus increasing its validity and reliability. However, this study is limited on the immunological marker assessed; it does not cover all immune profiles and cytokine responses related to SARS-CoV-2 infection. Moreover, we only assess the changes in gut microbiota profiling after probiotic supplementation (α and β diversity) in subsamples considering budgetary constraints, so it will reduce the generalisability due to the small sample size. Despite these strengths and limitations, this study could provide robust evidence to support an additional strategy to consider in high-risk individuals, including overweight and obese adolescents, to fight against COVID-19 infection.
Ethics and dissemination
The research protocol was reviewed and approved by the Ethics Committee of the Faculty of Medicine, Universitas Indonesia-Cipto Mangunkusumo Hospital (KET 763/UN2.F1/ETIK/PPM.00.02/2022: 1 August 2022). The study results will be disseminated in a reputable international journal (Q1 Scopus-indexed) and through presentations at scientific meetings and conferences with stakeholders. Authorship of any manuscript derived from this study will follow the authorship criteria defined by the International Committee of Medical Journal Editors.
Any alterations to the protocol that could influence the execution of the study, potential advantages for the subjects or subjects’ safety, such as modifications to study objectives, study design, patient population, sample sizes, study procedures, analysis of study outcomes or significant administrative aspects, will necessitate a formal amendment to the protocol. Prior to implementation, the principal investigator and the Ethics Committee of the Faculty of Medicine, Universitas Indonesia-Cipto Mangunkusumo Hospital (FKUI-RSCM) will agree on and accept the revision.
Administrative adjustments to the protocol refer to modest revisions and/or clarifications that do not impact how the research is to be carried out. The principal investigator will reach a consensus on these administrative modifications, and they will be recorded in a memorandum. Administrative changes will be reported to the Ethics Committee/Institutional Review Board.
Trial status
The recruitment phase began in October 2022. Thus far, 197 respondents have been recruited. The estimated end date for this study is June 2025.60
Supplemental material
Ethics statements
Patient consent for publication
Acknowledgments
We thank Professor Murdani Abdullah and Professor Dwiana Ocviyanti from the Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia, for their suggestions on the clinical aspect of the protocol. We also thank Dr. Anuraj H Shankar from Oxford University for his comments and suggestions on the use of immunity markers, application of data management and the statistical analysis plan for the study. We thank Chr Hansen, Denmark for providing the intervention products.
References
Footnotes
Contributors RA, KRE and MM drafted the research protocol and manuscript. All authors (including MJ) participated in the study design. RA coordinated the overall study in collaboration with JEB, EH, PSR, MM, WD, LD and TSN in conducting data collection at the study site. KRE and EP arranged the study administration. RA is the guarantor of the content of manuscript. All authors participated in, read and approved the final manuscript.
Funding This research is supported by grants from the Indonesian Endowment Funds for Education (LPDP), Ministry of Finance of the Republic of Indonesia which are administered by Universitas Indonesia (UI) under the PRIME Program (Grant No. NKB-14/UN2.RST/HKP.05.00/2023 and PRJ-120/LPDP/2021) on behalf of the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia. The research funder and manufacturer will not have roles in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Competing interests None declared.
Patient and public involvement Study subjects (adolescents) or the public will not be involved in the design, conduct, reporting or distribution plans of our research. Subject will be engaged in study implementation during screening and intervention periods.
Provenance and peer review Not commissioned; externally peer-reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.