Article Text
Abstract
Introduction Polypharmacy is associated with an increased risk of adverse patient outcomes across various settings, including inpatient care. To enhance the appropriateness of medication therapy management for patients during hospital stays, computerised interventions have shown promise with regard to patient safety. This study assesses whether the implementation of a clinical decision support system will optimise the process of inpatient medication therapy to prevent inappropriate medication use and thus promote patient safety.
Methods and analysis The intervention will be evaluated in a prospective, cluster-randomised controlled trial using a stepped-wedge design. The study will be conducted in 12 hospitals across Germany over a total period of 33 months. Patients will be treated according to the group status of the hospital and receive either standard care or the Transsektorale Optimierung der Patientensicherheit or trans-sectoral optimisation of patient safety intervention. The primary outcome is the combined endpoint of all-cause mortality and all-cause hospitalisation. Secondary endpoints are, for example, inappropriate prescriptions, utilisation of different health services, cost-effectiveness, as well as patient-reported outcome measures. Parameters describing the attitudes of patients and healthcare professionals towards the intervention and organisational change processes will be collected as part of the process evaluation. The primary endpoint will be evaluated using hospital and outpatient claims data from participating statutory health insurances at the population level. There are multiple secondary endpoints with data linkage of primary and secondary data at study participant level. Statistical analysis will make use of (generalised) linear mixed models or generalised estimating equations, taking account of independent covariables. All data analyses of the process evaluation will be descriptive and explorative.
Ethics and dissemination Data collection, storage and evaluation meet all applicable data protection regulations. The trial has been approved by the Ethics Committees of the University of Wuppertal and the Medical Association of Saarland, Germany. Results will be disseminated through workshops, peer-reviewed publications and local and international conferences.
Trial registration number DRKS00025485.
- Hospitals
- Polypharmacy
- Health & safety
- Medication Review
- Clinical Decision-Making
- Randomized Controlled Trial
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Hospitals
- Polypharmacy
- Health & safety
- Medication Review
- Clinical Decision-Making
- Randomized Controlled Trial
STRENGTHS AND LIMITATIONS OF THIS STUDY
The Transsektorale Optimierung der Patientensicherheit or trans-sectoral optimisation of patient safety study is a cluster-randomised controlled trial in a stepped-wedge design that will provide evidence on the effects of a clinical decision support system (CDSS)-based intervention for medication management in inpatient care on patient-relevant outcomes.
We hope to gain extensive insights into the effects by using different linked data sources; in particular, claims data from two statutory health insurance providers (SHIPs), self-reported patient data and CDSS software data.
In addition, the study will conduct a comprehensive process evaluation to provide insights into the implementation process, barriers to and factors facilitating implementation and embedding activities in routine care.
Due to convenience sampling at the hospital level, it may be that the hospitals participating in the study are already more receptive to medication therapy management (MTM) and aware of the technical possibilities for its realisation compared with other hospitals in Germany.
Since some hospitals have already established programmes for MTM, they start with better preconditions than others, potentially influencing various study endpoints. The comparability of study groups will be addressed by: (1) collecting data on the primary endpoint from a preobservational period and (2) collecting information on established measures in the hospital, allowing for the development and utilisation of appropriate control variables in the analysis.
Introduction
Polypharmacy, mostly defined as the concurrent use of at least five medications daily,1 is associated with an increased risk of adverse outcomes, including mortality, hospitalisation, adverse drug reactions, drug-drug interactions, medication non-adherence and high healthcare costs.1–6
The pooled prevalence of polypharmacy across various healthcare settings, regions and all medication classes is estimated at 37%,3 with a slightly lower estimate of 30% reported for Germany.4 This prevalence varies not only between countries and different healthcare settings, but also between population age groups. The prevalence of polypharmacy is in addition higher in inpatient settings compared with outpatient and community settings.3 4 7
During the transition from outpatient to inpatient care, there are often information gaps, potentially leading to avoidable harm. Adequate and safe decisions on diagnostics and therapy in hospital require full knowledge of a patient’s medical history and the outpatient treatment received.8–10 In addition, prescription errors are often not recognised on admission to hospital, and medication errors in hospitals occur frequently.11–15 Besides these, an inadequate transfer of information or inadequate coordination between different care providers after discharge from hospital can also lead to complications.9 16 Overall, hospitalisation is associated with an increased risk for patients’ medication safety.8 9 17
To enhance the appropriateness of medication therapy management (MTM) during hospital stays, computerised interventions have shown promise with regard to patient safety. Clinical decision support systems (CDSS), as one of the information technology (IT)-based interventions, are recognised as a promising approach to improve process-related outcomes, such as prescription and drug-drug interaction. However, there is limited evidence of the effects on patient-level outcomes such as readmission and mortality.18–24
The TOP study (‘Transsektorale Optimierung der Patientensicherheit’ or ‘trans-sectoral optimisation of patient safety’) will implement a complex CDSS-based intervention to optimise the process of inpatient medication therapy at admission, during the patients’ stay in hospital and at discharge, to prevent inappropriate medication use and thus promote patient safety. The term ‘complex intervention’ refers to the characteristics of the intervention itself (eg, different actors involved in the delivery and implementation of this multicomponent intervention). Complexity may also arise in our study from the interaction of the intervention with its context and the steps that need to be taken to implement the intervention.25
Objectives
The primary objective of TOP is to optimise the process of medication therapy for inpatients at admission, during the patients’ stay in hospital and at discharge, in order to improve MTM and to achieve related outcomes such as a cross-sectoral improvement in quality, safety, cost-effectiveness and coordination of medication therapy, as well as increasing patient autonomy and self-management skills for inpatients with polypharmacy. We will demonstrate whether this complex intervention can contribute to an improvement of care within a prospective, cluster-randomised controlled trial (C-RCT) and using the example of the statutory health insurance system in Germany.
Therefore, the TOP trial aims to:
Evaluate the effectiveness of the complex intervention: we will examine whether the intervention reduces mortality and readmissions in polypharmacy patients treated in hospital. Furthermore, we intend to assess the impact of the intervention on inappropriate prescriptions and severe and avoidable adverse drug events (ADEs), as well as the utilisation of outpatient emergency care.
Evaluate the cost-effectiveness of the intervention: we will determine incremental cost-effectiveness ratios (ICERs), in particular, the cost per avoided hospitalisation and/or death and the cost per quality-adjusted life year (QALY) from a payer’s perspective.
Evaluate patient-reported outcome measures (PROMs), such as health-related quality of life, and patient-reported experience measures (PREMs), such as experienced continuity of (pharmaceutical) care and patient satisfaction, with information about medication. These measures will be collected to complement the claims data-based assessment of effectiveness from the patient’s perspective.
Conduct a socioeconomic impact assessment to support sustainability planning for spreading and scaling up the TOP intervention.
Evaluate the implementation process and its outcomes in participating hospitals and assess factors hindering and facilitating the implementation of digital interventions.
Methods
This study protocol was written in accordance with the Standard Protocol Items: Recommendations for Interventional Trials reporting checklist (online supplemental file 1).26
Supplemental material
Study design
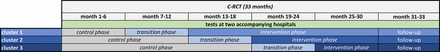
The intervention will be evaluated in a prospective, C-RCT using a stepped-wedge design (SWD) with an observation period of 33 months (30 months for recruiting +3 months for follow-up of the last patient to be included) running from August 2021 to April 2024. The trial is designed as a hybrid type-1 effectiveness-implementation study addressing, besides effectiveness as the primary focus, the implementation of the TOP intervention as well.27 A total of 12 hospitals will be randomly assigned to three clusters. Each cluster starts in a control phase. The intervention will then be introduced to each cluster with a time delay (ie, in steps), similar to a unidirectional crossover design (see figure 1). The patients will be treated according to the group status of the hospital and, depending on that group status, receive either standard care (control phase) or the TOP intervention (transition and intervention phase). The study design allows the recording and comparison of temporal effects as well as an intensive process evaluation.
Roll-out including follow-up of the cluster-randomised controlled trial (C-RCT) in a stepped-wedge design.
The intervention represents a novel process currently undergoing untested implementation within the hospital setting. Therefore, the two hospitals accompanying the study are responsible for testing and optimising the acceptability, appropriateness and feasibility of the intervention and its implementation. In addition, these test hospitals play a crucial support role for the hospitals involved in the C-RCT, helping them to successfully implement and embed the TOP intervention.
Setting and trial population
Study setting
The study will be carried out in 12 hospitals across Germany. These will include hospitals in different states, of different sizes, with different ownership structures and with departments relevant to the study (see Inclusion criteria). Each participating institution must give its consent by signing a cooperation agreement.
Inclusion and exclusion criteria
Hospital level
Hospitals can participate in the study if they are willing to hire a pharmacist for the duration of the study or to assign the study tasks to a suitably qualified member of staff. Furthermore, the hospital should be willing to assign staff to study-related tasks, such as informing and enrolling patients willing to participate, for the duration of the C-RCT. Hospitals that are already participating in a similar project will be excluded.
Staff level
Healthcare professionals (HCPs): the HCPs in the study will be pharmacists (ward or hospital pharmacists, depending on the existing structure of the hospital) and physicians from the participating departments who are directly or indirectly involved in the TOP intervention. Additionally, each participating hospital provides key persons (eg, project manager at the hospital, head of pharmacy and of the specialised department) as a responsible key informant on the implementation process.
Patient level
Included in the trial will be all patients aged 18 years and over and insured with participating health insurance providers (BARMER and AOK Nordost) who take at least five prescribed drugs and were initially hospitalised in a participating hospital’s department of internal medicine, geriatrics, visceral surgery, vascular surgery, cardiac and thoracic surgery, orthopaedics and trauma surgery, neurology or urology during the study period. Inclusion in the study will be consecutive. Patients undergoing oncological treatment will be excluded due to regular hospitalisation based on treatment cycles.
Recruitment
Hospital level
Hospitals are recruited nationwide, the aim being to recruit hospitals from several states, of varying size and, if possible, having the departments relevant to the study. The hospitals participating in the trial are based on a convenience sample.
Staff level
At the beginning of the trial, hospitals will designate key informants, who will be invited to participate in the data collection of key persons. In order to survey all HCPs (complete enumeration), medical and pharmaceutical staff will be recruited via key persons in the organisation concerned. They will also hand out the survey documents. Before taking part in data collection, all persons will receive written information about the surveys.
Patient level
Patients will be recruited at participating hospitals and will receive either standard care (control phase) or the TOP intervention (transition or intervention phase), depending on the hospital’s study phase. All patients who meet the inclusion criteria will be invited to participate in the TOP study by pharmacists during their admission as an inpatient. Patients will be provided with written information and must give their consent in order to participate in the study. Although patient recruitment in the hospitals started in August 2021 (month 1), participant enrolment did not start until November 2021. The start was delayed (compared with the start of the C-RCT) because the recruitment process and the transfer of patient information to the statutory health insurance providers (SHIPs) had to be introduced and established in the study hospitals, and the processes for checking patients' inclusion criteria and sending study documents to patients by the SHIPs had to be adapted. This had to be based on the existing structures and processes of healthcare practice and could therefore only be tested and adapted after the start of the C-RCT. The recruitment period ends on the last day of the intervention phase for all hospitals (month 30).
Randomisation
Randomisation takes place at the hospital level, that is, at the start of the project, all participating hospitals are randomly assigned to a cluster. Randomisation will be stratified based on the hospitals’ size (determined by number of beds and cases) to ensure that the numbers of control and intervention patients are balanced. There will be no randomisation at patient level, as it is assumed that the service providers in the hospitals will experience learning effects, which would then influence the treatment of patients from the control group.
Intervention and control
Description of the intervention
The TOP intervention is a complex intervention that focuses on intensified pharmaceutical care for patients on admission to hospital, during their inpatient stay and on discharge. MTM is a key component of the intervention and will be carried out electronically using CDSS-based software. The use of claims data from participating SHIPs, such as diagnosis, medication and healthcare service utilisation, facilitates the generation of CDSS insights, with additional parameter values such as renal function and body weight, along with dosage information and over-the-counter medication information, being entered by a pharmacist or physician. A physician’s confirmation of current prescriptions in the CDSS is also required to prevent outdated prescription data from being included in the medication review. Following the medical history, the CDSS evaluates the medication and generates alerts in three domains (drug-related, dose-related and drug-therapy related). The generated alerts are presented in a hierarchical order of severity, ranging from most serious to least serious and are categorised as ‘red’, ‘yellow’, ‘grey’, or ‘info’. The formulation of these warnings is informed by scientific and regulatory publications, as well as drug commissions. The process is carried out by pharmaceutical and medical professionals employed by the TOP-technology partner, using the World Health Organization – Uppsala Monitoring Centre algorithm (WHO UMC algorithm)28 and the criteria defined by the Drug Interaction Probability Scale.29 A detailed description of the several interdependent components of the intervention based on the TIDieR Checklist30 is given in online supplemental table 1 ‘Components of the the TOP intervention’ (online supplemental file 2).
Supplemental material
Implementation of the intervention
The intervention will be implemented in a time-lagged design in participating hospitals. Over the course of the study, each hospital will pass through the control period, the transition period and the intervention period. In the control phase, hospitals provide the usual care and initiate preparations for the intervention use, such as establishing technical connections or hiring required staff. The intervention is introduced in the hospitals for the first time during the transition phase. During this period, the intervention is tested under everyday conditions of the hospital. It is possible to make procedural adjustments to adapt the use of the intervention to the specific structures and processes of the respective facility. This transition period is followed by the intervention period, in which the intervention will be fully realised under everyday conditions. For validating effectiveness, it is crucial that certain actions of implementation are realised in the appropriate period and completed before transitioning into the next period.
Multicomponent implementation strategies will be carried out to enhance the implementation process and the outcomes (eg, fidelity) of the TOP intervention in routine care. The initial implementation strategies in TOP cover the elements of providing interactive assistance, developing stakeholder interrelationships, training and educating stakeholders, engaging consumers, using financial strategies, adapting and tailoring the TOP intervention to the context and using evaluative and iterative strategies based on the pragmatic implementation strategy reporting tool.31 For details of the implementation strategies, see online supplemental table 2 ‘Implementation strategies of TOP’ (online supplemental file 3).
Supplemental material
Control group
In the control phase, hospitals provide care according to their current standards. As we do not explicitly include MTM naive hospitals, it cannot be ruled out that hospitals may provide MTM or use CDSS if they have already established these elements as part of their standard care process.
Outcome assessment
Primary outcome
The primary outcome, based on claims data from participating SHIPs, is the combined endpoint of all-cause mortality and all-cause hospitalisation in polypharmacy patients, 3 months after discharge.
Secondary outcomes
The primary endpoint is followed by several secondary endpoints regarded as significant for the overall success of the intervention. In detail, these include the effectiveness and cost-effectiveness of the intervention regarding the following aspects:
Percentage/proportion of patients who receive inappropriate prescriptions and percentage/proportion of patients who suffer from severe and avoidable ADEs at the hospital or within 3 months postdischarge (based on claims data).
Utilisation of different health services such as emergency care (based on claims data).
Cost-effectiveness and cost-utility of the intervention compared with standard care (based on claims data and survey data).
PROMs and PREMs will be collected by questionnaire at two measurement points: shortly after discharge from hospital (t0) and approximately 90 days after discharge (t1). PROMs and PREMs will include ADEs (Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE)32; t0 and t1), experienced continuity of (pharmaceutical) care (Patient Continuity of Care Questionnaire33; t0 and t1), satisfaction with information about medicines (german version of the Satisfaction with Information about Medicines Scale (SIMS-D)34; t0 and t1), adherence (german version of the Medication Adherence Report Scale (MARS-D)35; t0 and t1), patient enablement/empowerment (Generic Questionnaire for Measuring Patient Enablement36; t0 and t1), patient safety (Patients’ Perceptions of Safety Culture Scale37; t0) and health-related quality of life (german version of the Veterans Rand Six Dimension (VR-6D)38 and the 5-level EQ-5D version (EQ-5D-5L)39; t0 and t1).
Costs and benefits (financial, resources/time and intangible) of the intervention at the level of stakeholders and the service overall (based on claims data, software routine data and survey data).
Process evaluation
A process evaluation will be conducted to understand the intervention effects of complex interventions such as TOP and to identify their potential for generalisability and possible improvement.40 The process evaluation will therefore involve the scientific monitoring of the intervention throughout the duration of the planned C-RCT and address questions of a process-descriptive (eg, how is the intervention implemented in healthcare practice? What factors inhibit or promote the implementation of the intervention?) or organisational-change nature (eg, how the intervention influences the structure, workflow or culture within the participating hospitals?). The process evaluation will assess how and why the intervention works (or does not work) in the specific context where it is being applied. The evaluation will follow the Medical Research Council’s recommendations for process evaluations of complex interventions,41 be guided by the framework established by Grant et al42 for monitoring C-RCTs, identify effect-modifying factors and focus on exploring mechanisms of impact. The process evaluation of TOP will include different data collection methods (written questionnaires, interviews, document analysis of field notes and software data) from different target groups (patients, pharmaceutical and medical staff, key person at the hospital, for example, management, project manager). Data will be collected on context (eg, organisational readiness for implementing change, pharmaceutical and medical staff acceptance and use of the technology and previous MTM experience of patients), recruitment (eg, reasons for participation, implementation practices and resources of hospitals and number of patient consents), implementation (eg, appropriateness, feasibility and acceptability of the intervention by pharmaceutical and medical staff and perception and evaluation of intervention components by enrolled patients, such as contact with the pharmacist), mechanism of impact (eg, use of intervention in work routine, patients’ attitudes and experiences of the TOP intervention) and effectiveness (eg, organisational expectations of the intervention met, hospitals’ intentions to continue with the intervention) throughout the duration of the C-RCT. The data collection time points at the organisational level will be determined by the hospital’s affiliation with one of the three switching cohorts. More detailed information on the data collection of process evaluation is given in online supplemental table 3 ‘Data collection of process evaluation’ (online supplemental file 4).
Supplemental material
Data collection and management
Data collection
Secondary data/claims data from participating health insurance providers
Claims/routine data from August 2021 to April 2024 will be used in the analysis. Data collection is therefore longitudinal, and data will be available for the duration of the C-RCT, as well as the preobservation period so as to establish a baseline for the primary endpoint.
The required claims data from participating SHIPs are specified in a coordinated minimal data set. Variables included in the dataset are sociodemographic patient data (sex, age, insurance status and the reason insurance coverage ended), outpatient diagnoses and outpatient services (International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) diagnoses and services according to the physician’s fee scale), medication (pharmaceutical registration number, Anatomical Therapeutic Chemical (ATC) code, duration of the therapy (Defined Daily Dose (DDD) and costs), inpatient data (start and end date of each hospitalisation, admission and discharge diagnoses, secondary diagnoses, operation and treatment procedures and costs), long-term nursing care (start and end date, level and place of care, costs and type of service), incapacitation for work (ICD-10, start and end and costs) and ambulance services (start and end).
Primary data
Primary data will be collected from patients treated with the intervention (transition and intervention phase) as well as those who have confirmed their willingness to participate in the data collection (control phase) and are able to consent. Data will be collected through a questionnaire sent by post, shortly after discharge from hospital (t0) and approximately 90 days after discharge (t1). All survey participants who have agreed to be contacted by the health insurers after hospitalisation and who meet the inclusion criteria of the survey will be contacted. This procedure was chosen to ultimately achieve a response rate of at least 25% of all study patients. The questionnaires will be delivered by the health insurance providers and will be returned to the University of Wuppertal. Once the pseudonymised questionnaires arrive at the University of Wuppertal, they will be scanned in.
To monitor implementation status and possible obstacles to the implementation of the intervention, the hospitals will be asked to report any unexpected events every 3 months. These are events at the individual level (eg, illness of the staff responsible), ward level (eg, staff shortages) and hospital level (eg, strike). The survey will be sent to the contact persons in the hospitals via e-mail.
Data from the software solution
For the data from the software solution for evaluating the secondary endpoints (medication plans) and user behaviour (process evaluation), a minimal dataset is coordinated with the operator of the software, enabling the corresponding evaluations. Data from the software solution include, for example, information about boxes clicked within the software by the pharmacists, such as ‘Medication therapy recommendation set’, ‘Pharmaceutical discharge interview done’, ‘Printed handout on medication therapy given to patient’, the medication the patients take, interactions and alerts.
Data from process evaluation
The collection process for the data from process evaluation will depend on the type of data collection. Qualitative data will be collected through face-to-face or telephone interviews from a subsample. A structured guide will be used during the interviews. All interviews will be recorded and subsequently transcribed according to appropriate guidelines.43 In the case of written surveys of staff (medical, pharmaceutical), the aim is to conduct a full survey. The questionnaires will be created using Teleform and sent to the HCPs by the responsible evaluation partner (University of Wuppertal). Delivery of questionnaires to patients (t0 and t1) for collecting data for the process evaluation is identical to the data collection process for primary data. All completed questionnaires will be sent back to the responsible evaluation partner (University of Wuppertal). Once received, the pseudonymised questionnaires will be scanned and digitised.
For the document analysis, field notes used during the introduction and implementation of the intervention or created during the evaluation will be collected. They may include, for example, training protocols and hospital implementation guides. Hospitals will provide copies of documents to the responsible evaluation partner (University of Wuppertal) for analysis or grant access for data extraction. The method of document analysis will help identify, for example, factors supporting or hindering the implementation process.
The data collected, including interview transcripts, questionnaire responses and documents, will be processed using established software tools commonly used in the social sciences. These tools include SPSS, R and MAXQDA, which allow for a thorough analysis and interpretation of the data.
Data management
The evaluation consists of two study sections, with study section 1 using only claims data from participating SHIPs at the population level and study section 2 using claims data from participating SHIPs, primary data and medication plan data from the software used, all at the study participant level (see figure 2).
Study sections. ITT, intention-to-treat;SHI, statutory health insurance; MTM, medication therapy management.
The primary endpoint will be evaluated using claims data from the participating SHIPs at the population level (all potential patients in the hospital, intention-to-treat and study section 1). Both SHIPs will select records for the relevant observation periods in participating wards of participating hospitals, based on the inclusion criteria of a minimum age of 18 and being prescribed three or more drugs. To estimate a baseline of MTM measures, characteristics of the primary endpoint in participating hospitals will be calculated from a preobservation period using this dataset.
The secondary endpoint is based on multiple endpoints with a linkage of primary and secondary data (individual study participant level, per-protocol and study section 2). The secondary endpoint will be divided into the analysis of patients enrolled and treated and patients enrolled in the control phase and receiving standard care. For patients in the control phase, claims data, primary data at the individual level (patients) and primary data at the organisational level (hospital) will be linked. The linkage of the primary data at the individual level, the software data and the claims data will be done using a pseudonym for each patient. An institutional pseudonym will be assigned to the primary data at the organisational level, collected for the formative evaluation/process evaluation for matching with the hospital concerned.
Analysis
Sample size
According to a preliminary analysis based on BARMER routine data, the incidence of readmission or death within 90 days from initial hospitalisation is 32.9% (24.81% readmission <90 days; mortality 2.66% in hospital, 5.43% <90 days after discharge). Assuming a 15% reduction of the primary combined endpoint to 27.97%, an α-error of 0.05 and a power of 1-β=0.80, the underlying regression model yields a sample size of 146 treated patients or cases per hospital per time interval (quarterly period). With 12 hospitals participating over eight quarters (30 months excluding the transition phase), the total number of patients treated is aimed to be 14 016 (7300 for the control phase and 6716 for the intervention phase). An expected dropout rate of 40% increases the sample size to 23 328, with 243 patients to be recruited per hospital per quarter. To evaluate the implementation appropriately in the process evaluation, an additional 5832 patients should be recruited for the transition phase in the 12 hospitals. This sample size was calculated using an intraclass correlation coefficient of 0.05, which had been determined in the preliminary analysis of the BARMER data. The dropout rate is based on a previous study within the outpatient setting having a similar intervention and the same primary endpoint based on claims data from participating SHIPs.44
Analysis of primary and secondary outcome parameters
The primary objective of this study is to determine whether this complex intervention reduces the combined endpoint of all-cause mortality and all-cause hospitalisation in adult patients with polypharmacy, 90 days postdischarge.
The evaluation strategy for the primary endpoint is based on study section 1, including all patients insured with participating health insurance providers who were hospitalised in a participating hospital during the study period and fulfil the inclusion criteria. In this way, not only are the effects of the intervention on patients treated with this new form of care taken into account, but also access to the intervention and spill-over effects regarding the treatment of non-participating patients. In subordinate analysis, group comparisons will be made, in which selective contract participants are to be compared with control group patients as a subgroup.
Statistical analysis will be conducted for primary and secondary endpoints (study section 1 and study section 2) at cluster level, in the form of within-cluster and between-cluster analyses. Inappropriate prescribing is operationalised using the PRISCUS 1 list, the Fit fOr The Aged (FORTA) list and the negative drug interactions of the ‘choosing wisely’ (‘Klug entscheiden’) initiative. Prescriptions are available for outpatient data. Avoidable ADEs are operationalised according to Stausberg and Hasdorf.45 ICD-10 diagnoses are available for outpatient and inpatient cases. First, the primary and secondary endpoints will be analysed descriptively. The statistical analysis will use (generalised) linear mixed models46 or generalised estimating equations,47 48 taking account of independent covariables (eg, age and gender). The multilevel structure will also be accounted for by fixed time effects and random effects for clusters. The use of these statistical models enables the estimation of intervention effects through a binary covariate, where 1 represents the intervention group (clusters in the intervention period) and 0 denotes the control group (clusters in the control period). The results of the survey of unexpected events will be included in the analysis as confounders to control for possible effects of these events. For the analysis of study section 2, medication plan data from the software will be included in addition to the confounders available in the claims data. Analyses will be adjusted for multiple testing by Bonferroni correction.
Primary patient level data analysis will be descriptive and exploratory. The statistical analysis will follow the procedures used for the routine data.
Cost-effectiveness analysis
The health economic analysis will be conducted from a third-party payer perspective, which is the perspective of the SHIPs in Germany. The ICER will be calculated by dividing the difference in costs by the difference in health benefit of the intervention compared with standard care. The analysis of all reimbursed direct healthcare costs will be based on health insurance claims data comprising healthcare resource utilisation regarding inpatient care, outpatient care, rehabilitative care, pharmaceuticals, therapeutic devices, non-physician specialist services, nursing (home) care and patient transport services and sick pay. Intervention-related costs will also be included. Benefits of the intervention will be measured by the primary outcome (hospitalisation and/or death) and the secondary outcome of health-related quality of life. Hence, the cost per avoided hospitalisation and/or death (cost-effectiveness analysis (CEA)) and the cost per QALY (cost-utility analysis (CUA)) will be analysed.
The CEA will be based on the population level (see section Data collection). However, for the CUA, data obtained by the EQ-5D-5L39 collected in the C-RCT will be used to calculate QALYs using the German value set.49 Thus, the CUA will be based on a reduced sample.
Socioeconomic impact assessment
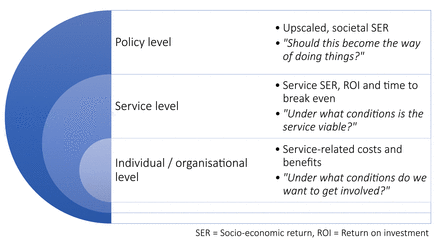
In order to analyse the costs and benefits of the intervention at the level of stakeholders and the service overall, a socioeconomic impact assessment (SEIA) will be conducted. SEIA is a formative evaluation to support sustainability planning of the intervention for transfer into regular care which aims to answer questions on the three levels (see figure 3).
Methodologically, SEIA is based on cost-benefit analysis as defined by Drummond50 and the recommendations of UK HM Treasury,51 the Federal Government Commissioner for Information Technology52 and the White House Office for Management and Budget.53 An already established framework and associated evaluation software developed for business model development for IT-based utility services will be used.54
Socioeconomic impact assessment—addressed levels. [SER = Socio-economic return, ROI = Return on investment]
Process evaluation
All data analyses of the process evaluation will be descriptive and explorative. The analysis of qualitative data material will be either content analysis or a qualitative-descriptive analysis. In analysing the interviews, a deductive procedure will be followed initially, in which paraphrases from the interviews are assigned to themes and subthemes of the underlying frameworks or theories of the respective data collection (eg, Consolidated Framework for Implementation Research, normalisation process theory). Within the themes and subthemes, the content will be processed inductively. The analysis of the questionnaire and software data will be descriptive and exploratory.
Patient and public involvement
This protocol was developed without patient or public involvement.
Discussion and limitations
The present study investigates a complex CDSS-based intervention to optimise the process of inpatient medication therapy at admission, during the patients’ stay in hospital, and at discharge, to prevent inappropriate medication use and thus promote patient safety. The method used is a hybrid type-1 effectiveness-implementation C-RCT with a stepped-wedge design addressing, besides effectiveness as the primary focus, the implementation of the TOP intervention as well. In the transition and intervention phase, patients will be offered intensified pharmaceutical care at admission to hospital, during their inpatient stay and on discharge. MTM is a key component of the intervention and will be carried out electronically using CDSS-based software. During the control phase (control group), patients will receive care according to respective hospitals’ standard.
Different data sources are linked to gain extensive insights into the effects of the intervention, in particular claims data from two SHIPs, self-reported patient data and CDSS software data. The comprehensive process evaluation will contribute to a deeper understanding of how different components of the interventions will work and will provide insights into the implementation process, barriers to and factors facilitating implementation.
This study has limitations. As organisational and structural changes are being addressed, blinding is not possible. In addition, language barriers may also reduce the response rate, as the questionnaire will only be available in German. Due to convenience sampling at hospital level, it may in addition be that the hospitals participating in the study are already more receptive to MTM and aware of the technical possibilities for its realisation compared to other hospitals in Germany. Since some hospitals have already established programmes for MTM, they start with better preconditions than others, potentially influencing various study endpoints.
Trial status and registration information
Hospital recruitment began with the project’s start in October 2020. Start of the C-RCT was August 2021, while the first participant enrolment was planned for 15 September 2021 and was started in November 2021. The last patient in will be at the end of January 2024, and the 3-month follow-up will be accordingly completed at the end of April 2024. Data collection will be completed by the end of August 2024. Analyses will be completed in September 2024.
Registration of the trial was initiated before the start of the C-RCT (August 2021), displayed on the public website after the start of the C-RCT (09 September 2021) but before the date of the first enrolment of participant (planned for 15 September 2021, with the first valid enrolment in November 2021).
Ethics and dissemination
The TOP study was approved by the Ethics Committees of the University of Wuppertal (no. MS/AH 201028) and the Medical Association of Saarland, Germany (no. Ha 37/21). In case of important modifications of the protocol, the aforementioned Ethics Committees and the funding institution will be informed immediately.
Written informed consent will be obtained from the managers of each participating hospital through their signing a supply contract. HCPs will receive comprehensive information on the study before getting involved in data collection. The staff can stop data collection at any time if they wish.
Written informed consent will be obtained from all participating patients if they decide to get involved in the study during their hospital stay (individual study participant level, study section 2). A two-stage process has been developed for consent to participate in the study. Consent to participate in the study is only valid after the participant has read the information, had the possibility to ask questions and signed the informed consent for participation in the study (during the hospital stay) and the informed consent for the scientific monitoring and evaluation of the study (after the hospital stay). For model consent forms, see the online supplemental file 5. Any participant may withdraw their consent at any time. The data of study section 1 (claims data at the population level) are provided on the grounds that it would be unreasonable to obtain the consent of all patients insured with the participating SHIPs. These grounds include, among other things, the relevance of the study to the general population and the risk of bias due to selection effects when consent is obtained.
Supplemental material
A declaration of consent permitting data access is a prerequisite for retrieving patient-related information from the health insurance providers. The software documents which registered user of the hospital information system has retrieved or processed information, on which patient and at what time.
When the software is installed, a key encrypted by a fixed code and managed in the software is generated, that is, only the software knows the patient-related data. Only authorised hospital staff can access the data stored. Neither the health insurance nor the software provider can view the treatment data.
BARMER and the hospitals accompanying the study will be responsible for monitoring the trial and, in particular, for recruiting hospitals and supporting patient recruitment and the implementation of the intervention by the study hospitals. The monitoring of BARMER includes, for example, performing random checks of whether a valid declaration of consent exists for insured patients whose health insurance data have been retrieved for treatment support. The steering committee, consisting of members of the TOP study group, will meet via telephone conferences two times a month to review the progress of the study and to make decisions within the framework of the study if necessary. The participating SHIPs, the study hospitals and the software provider will be responsible for providing data to the evaluation team. A designated advisory board will provide advice on the design, conduct and analysis of the trial. The data monitoring committee, consisting of members of the SHIPs, the hospitals accompanying the study, the software provider and the evaluation team, will be independent of the institution funding the study and of competing interests.
Results of the study will be disseminated through publications in international, peer-reviewed journals and conference contributions. The reporting of the results will adhere to the Consolidated Standards of Reporting Trials Statement extension for cluster-randomised trials.55
Ethics statements
Patient consent for publication
Acknowledgments
We would like to thank all hospitals and patients for their participation in the study. We appreciate the support of the hospitals that accompanied the trial and the project management support provided by BARMER.
References
Footnotes
Collaborators TOP study group: Till Beckmann (BARMER), Lara Düvel (BARMER), Susanne Dolfen (AOK Nordost), Nathalie Sinnen (AOK Nordost), Carolin Polte (AOK Nordost), Olga Resch (AOK Nordost), Nicole Faulenbach (AOK Nordost), Hannah Britz (Klinikum Saarbrücken gGmbH), Beate Halex (Klinikum Saarbrücken gGmbH), Sofie May (University Hospital Münster), Matthias Schilling (University Hospital Münster), Simone Grandt (RpDoc Solutions) and Christof Shelian (RpDoc Solutions).
Contributors RB drafted the first version of the manuscript with input from JK-N and SM. Critical revision of manuscript for important intellectual content: SS, DL, AP, IM and JK-N. SM, DL, AP, DG, CK, IM, WG and JK-N are responsible for study concept and design. AD is the study director. Acquisition of data: SM, SS, DL, AP, PI, IM, WG, JK-N and the TOP study group. Analysis and interpretation of data will be performed by SM, SS, DL, AP, IM, WG and JK-N. PI is responsible for data management and the trust centre. JK-N is the chief investigator of the study and is also the guarantor. All authors reviewed the paper and read and approved the final manuscript.
Funding This study was funded by the Innovation Fund of the German Federal Joint Committee (G-BA) (grant: 01NVF19018).
Competing interests SM, DL, PI, SS, CK, AD, KCR, IM, WG and JK-N report grants from the German Federal Joint Committee during the conduct of the study. DG reports grants from BARMER during the conduct of the study and a family member of DG works for and holds shares of IT company involved in the project. SG works for and holds shares of IT company involved in the project. All other authors have no competing interest to declare.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.