Article Text
Abstract
Introduction Postoperative delirium occurs in up to 40% of older surgical patients and has been associated with prolonged hospital stays, long-term cognitive impairment and increased 1-year postoperative mortality. Postoperative sleep disturbances may increase the risk of delirium, but studies investigating pharmacotherapies to improve postoperative sleep to prevent delirium remain limited. Suvorexant is a selective antagonist of orexin 1 and 2 receptors and is approved for insomnia pharmacotherapy by the Food and Drug Administration. It has the potential to improve postoperative sleep and reduce postoperative delirium rates, but randomised controlled trials (RCTs) are needed to determine the efficacy of postoperative suvorexant administration. The REPOSE study (reducing delirium by enhancing postoperative sleep with suvorexant) is a single-centre, randomised, double-blinded RCT that aims to evaluate the efficacy of suvorexant in increasing total sleep time (TST) and decreasing delirium severity in older patients undergoing non-cardiac surgery.
Methods and analysis REPOSE will enroll 130 patients (aged ≥65 years) undergoing non-cardiac surgery with a planned postoperative inpatient stay. Participants will be randomised to receive 20 mg oral suvorexant or placebo nightly on postoperative nights 0, 1 and 2. The primary endpoint is TST on the first postoperative night, as measured using an electroencephalography headband. The secondary endpoint is peak postoperative delirium severity as measured by the 3-minute diagnostic interview for the confusion assessment method severity scores. Primary endpoint data will be analysed with a two-sample t-test using an intent-to-treat approach to compare TST on the first night that a patient received a study drug dose. Secondary and exploratory endpoint data will be analysed using two-sample t-tests between groups.
Ethics and dissemination Ethical approval was obtained from the Duke Institutional Review Board (protocol #00111869). Results of the REPOSE study will be published in a peer-reviewed journal and presented at academic conferences. Trial data will be deposited in ClinicalTrials.gov.
Trial registration number NCT05733286.
- Delirium
- SLEEP MEDICINE
- Clinical Trial
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study uses electroencephalography headbands to determine whether postoperative suvorexant administration increases postoperative total sleep time.
Trained staff members assess the secondary outcome of delirium severity twice daily using the 3-minute diagnostic interview for the confusion assessment method delirium assessment.
This study will not provide information on whether suvorexant improves sleep following hospital discharge, because the drug and sleep measurements are stopped upon hospital discharge.
The single-centre setting limits the generalisability to other populations due to potential site-specific biases.
Introduction
Postoperative delirium is a disorder characterised by acute confusion, impaired attention, disorganised thinking and disturbances in consciousness, and it typically occurs in the first 3 days following surgery.1 Postoperative delirium affects up to 40% of older patients undergoing surgery and is associated with increased hospital length of stay, long-term cognitive decline, Alzheimer’s disease and related dementias and increased 1-year postoperative mortality.2–5 Although delirium is associated with poor postoperative outcomes, there are few interventions that prevent delirium, in part due to the difficulty of addressing unmodifiable delirium risk factors, such as older age and baseline cognitive impairment.6
One potentially modifiable risk factor for delirium is postoperative sleep disturbance.7–10 Sleep is a fundamental physiological process that influences cognition, emotional well-being, immune function and homeostasis.11–14 Following surgical procedures, patients frequently experience sleep disruptions due to excessive hospital noise and light, postoperative pain and the effects of medications administered during the perioperative period.15–17 Thus, strategies that mitigate postoperative sleep disturbances and promote sleep hygiene may decrease the risk of postoperative delirium.15 18 19 Additionally, the appropriate administration of pharmacological sleep aids may help prevent postoperative delirium. However, few studies have widely investigated pharmacological sleep aids for postoperative delirium prevention, in part because some sedating pharmacological sleep aids may increase the risk of delirium. For instance, benzodiazepines, often given for sedation in the intensive care unit (ICU), exacerbate postoperative delirium in older patients in the ICU.20–24 Consequently, recent guidelines from the American Geriatric Society discourage the use of benzodiazepines in older adults.25
Suvorexant is an emerging alternative pharmacotherapy for treating sleep disturbances. Suvorexant received Food and Drug Administration approval in 2014 for the treatment of insomnia and acts by blocking orexin receptors. Orexin is a wake-promoting neuropeptide pivotal for sleep regulation.26 27 By blocking orexin receptors, suvorexant improves both sleep onset latency and wake after sleep onset. Interestingly, suvorexant administration acutely reduces cerebrospinal fluid phosphorylated tau levels, an Alzheimer’s disease biomarker, which suggests that blocking the orexin pathway may affect Alzheimer’s disease-related pathology.28–30 Additionally, suvorexant increases total sleep time (TST) in older adults with probable Alzheimer’s disease dementia. Thus, suvorexant may be an ideal candidate for postoperative sleep pharmacotherapy in older patients undergoing surgery who are at high risk for delirium.31
To evaluate suvorexant’s efficacy in increasing postoperative sleep and reducing postoperative delirium severity, we are conducting a single-centre, double-blinded, placebo-controlled, randomised trial. Our primary hypothesis is that postoperative suvorexant administration increases TST in older patients undergoing surgery. Secondarily, we hypothesise that suvorexant administration decreases postoperative delirium severity. Our study aims to provide valuable insights into the potential therapeutic role of suvorexant to improve postoperative sleep and reduce delirium severity in older patients undergoing surgery.
Methods and analysis
Study design
This randomised, double-blinded, placebo-controlled trial will be conducted at a single centre at Duke University Medical Center in Durham, North Carolina, USA. The trial began on 28 June 2024 and is estimated to finish enrollment in late 2025. The study design schedule is outlined in table 1 and is approved by the Duke Institutional Review Board (IRB). Participants will be enrolled and randomised to intervention until 130 participants have completed the primary outcome data for analysis.
Schedule of study events
Study population
This study will enroll patients undergoing non-cardiac surgery with a planned postoperative inpatient overnight stay, who will undergo randomisation and receive at least one dose of the study drug. Inclusion and exclusion criteria are listed in table 2. Exclusion criteria include factors that affect suvorexant administration safety, pharmacokinetics or metabolism/excretion and the ability to safely wear the electroencephalography (EEG) headband (eg, intracranial surgery). Given the complexity of patient and surgical factors that could affect the safety of suvorexant administration or study assessment, we will also sparingly exclude some cases based on the principal investigator’s judgement. The reasons for these principal investigator determinations will be provided in the results of the study.
Inclusion and exclusion criteria of the REPOSE study with rationale
Potential candidates will be identified and screened for eligibility using the electronic health record. Eligible participants will be contacted through either an automated MyChart message or a phone call by a designated study team member using the IRB-approved phone script. All subjects will be informed of the purpose, procedures and intent of the study and be provided with a consent form (see online supplemental file 1) prior to enrollment. Informed written consent will be obtained before the subject initiates any study activities or begins any screening procedures that are not considered standard patient care activities. All sexes, races and ethnicities will be recruited.32
Supplemental material
Intervention
At least 130 participants will be randomised to receive suvorexant (20 mg) or a matched placebo for the first 3 postoperative nights while in the hospital. Patients will be administered suvorexant or placebo orally unless they are not able to take medications by mouth due to difficulty swallowing, in which case the medication can be administered via an indwelling nasogastric or gastrostomy tube if one is already present and is usable. Patients will be assigned to blinded treatment groups through stratified permuted block randomisation in a 1:1 ratio for suvorexant or placebo. Stratification will be based on age (≥70 years and <70 years of age) and sex (male vs female) to ensure balanced age and sex between study groups. The administration will occur starting on postoperative day 0 through postoperative day 2, between 20:00 and 22:00. After an order for the study drug is placed, the investigational drug pharmacy will be responsible for randomising, barcoding and labelling the study drug. The study team will then deliver the study drug to the bedside nurse, who will scan the study drug into the electronic medical record after administration in order to accurately track the study drug administration for each participant. The study team and participants will remain blinded to treatment allocation throughout this process.
Because suvorexant is primarily metabolised by CYP3A, the primary clinical team for enrolled subjects will be advised to avoid ordering moderate or strong CYP3A inhibitors.33 If recent exposure (<12 hours) to a moderate CYP3A inhibitor is noted in the electronic health record, the study drug dose will be halved, and if the subject is exposed to a strong inhibitor (within 12 hours of study drug administration), the study drug will not be administered. However, eligibility for future doses on subsequent postoperative nights is retained if criteria are met. Non-sedating sleep aids like melatonin are allowed anytime, while sedating sleep aids (table 3) are generally restricted on nights of the study drug. To allow for the standard of care treatment of in-hospital insomnia, patients are permitted sedating sleep aids for persistent sleeplessness 1 hour after study drug administration, up until midnight. The use of rescue sedating sleep aids for sleeplessness will be reported between placebo and suvorexant. Patients who receive rescue sedating sleep aids will not be excluded from the primary analysis.
Sedating sleep aids restricted in the REPOSE study
Several strategies will be used to promote retention and completeness of follow-up data. First, in the case that the study drug is discontinued for any reason after randomisation, participants will still be encouraged to remain in the study to continue the other study-related assessments. Second, if the participant is withdrawn from the study for any reason, every effort will be made to continue collecting data from the electronic medical record.
In the case that three unexpected, related serious adverse events are reported in this study, the enrollment of new participants will be halted, and an unblinded staff statistician will perform analyses to determine whether these related serious adverse events are associated with the suvorexant or placebo group. This information will then be used by the designated medical monitor to decide whether to continue the study.
Study outcomes and assessment
Primary endpoint
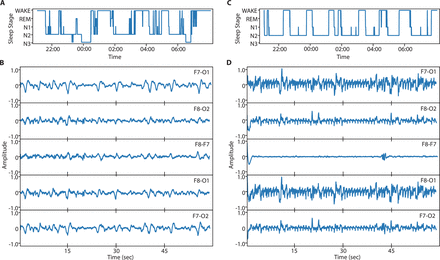
The primary endpoint is TST, which is defined as the amount of time spent sleeping during the lights-out period (21:00–6:00) on the first night after surgery that the patient receives the study drug. TST will be assessed using EEG during postoperative nights 0 through 2 or until hospital discharge, whichever occurs first. A headband with frontal electrodes, along with a 3D accelerometer, will be used to record EEG data, and the data will be saved for later analysis to determine sleep stage and TST (figure 1).34 This sleep EEG will allow for the determination of TST despite the frequent interruptions of sleep that occur postoperatively in the hospital wards. The headband is designed to be less invasive than standard polysomnography so that study subject sleep is minimally impacted by EEG monitoring, and EEG recording adherence is optimised.
Electroencephalography (EEG) recordings of relatively acceptable sleep architecture (A and B) and relatively disturbed sleep architecture (C and D). (A) Sleep architecture is preserved in one night of interrupted sleep, with sleep stages N2 and N3 observed alongside short periods of Rapid Eye Movement sleep (REM) in a hypnogram constructed from EEG data. (B) N3 sleep is captured in a 60 s interval across five channels, derived from pairings of the four electrodes (F7, F8, O1 and O2). (C) Sleep architecture is disturbed in one night of interrupted sleep, as suggested by observation of N2 sleep without N3 or REM stages. (D) N2 sleep is recorded across the five channels in a 60 s period of irregular sleep.
Sleep scoring of the EEG will be performed by trained sleep technologists overseen by sleep medicine physicians. Each 30 s epoch will be scored according to the American Academy of Sleep Medicine Manual for sleep stage and associated events.35 In epochs with poor EEG quality, where sleep stage is indeterminate, sleep presence will be imputed based on adjacent epoch staging. In epochs where the adjacent epoch staging is also indeterminate, the accelerometer data will be used to estimate whether the patient was asleep or awake.
Secondary endpoint
The secondary endpoint is peak postoperative delirium severity scores up through postoperative day 5 or discharge. The 3-minute diagnostic interview for the confusion assessment method (3D-CAM) will be used to assess delirium in patients who are able to communicate verbally.36–38 The 3D-CAM measures delirium severity through attention and cognitive assessments, including orientation items, short-term recall and digit span tasks. It then scores the presence of key delirium features, such as acute or fluctuating course, inattention, disorganised thinking and altered level of consciousness.38 In non-verbal or intubated patients, the confusion assessment method for the ICU (CAM-ICU) will be used instead.39 Assessment will first occur in the afternoon, prior to 21:00, on postoperative day 0. Subsequently, from postoperative days 1 to 5, assessments will be systematically documented two times per day until the conclusion of postoperative day 5 or hospital discharge, whichever occurs first. The first assessment will be administered prior to 12:00, and the second assessment will be administered between 12:00 and 21:00.
Exploratory endpoints
Sleep architecture and other sleep features
Using the EEG headband data, differences in postoperative sleep architecture (including stages 2 and 3 Non-REM sleep and REM sleep) will be compared in patients who received suvorexant compared with those who received placebo using two-sample t-tests. No multiple comparison correction for multiple sleep stages is planned because these are exploratory analyses. The average TST over all nights that subjects receive the study drug will also be compared using two-sample t-tests.
Subjective sleep quality
To assess subjective sleep quality, the Richards-Campbell Subjective Sleep Quality Questionnaire will be administered daily from postoperative day 1 until postoperative day 5 or hospital discharge, whichever occurs first. The total subjective sleep quality score will be compared between placebo and suvorexant groups using a two-sample t-test.
Sleep-related impairment in sustained attention
Sleep deprivation results in a decreased ability to sustain attention, which can be measured with the 5 min psychomotor vigilance task (PVT).40 The PVT measures simple reaction times to a visual stimulus over 5 minutes to assess for slowed responses and lapses (ie, failed response to visual stimuli).40 41 Response times, speed and lapses will be compared between suvorexant-treated and placebo-treated groups to see if postoperative nightly suvorexant has an effect on these sustained attention measures. Using the NASA PVT+ application on an Apple iPad, the PVT will be collected before surgery and daily on postoperative day 1 and ending on postoperative day 3 or hospital discharge, whichever occurs first. Analyses will also examine preoperative-to-postoperative change in sustained attention measures to adjust for preoperative performance variability between subjects.
Delirium incidence and duration
The incidence and duration of postoperative delirium in treatment groups will also be reported. Although this study does not have adequate power to detect differences in delirium incidence, these data could be useful to guide future larger studies or in a meta-analysis of all studies of suvorexant and delirium.
Pupillary unrest in ambient light
Pupillary unrest under ambient light is an index of spontaneous pupil fluctuations that occur secondary to the activity of the locus coeruleus, an important brainstem nucleus involved in the maintenance of wakefulness and attentional control.42 43 Decreased wakefulness has been associated with decreased pupillary unrest under ambient light, suggesting that pupillary unrest in ambient light is a marker of sleep deprivation-related alterations in wakefulness and attention. Here, infrared pupillometry will be used to measure pupil diameter fluctuations under ambient light conditions both before surgery and daily up through postoperative day 3.44 We will compare postoperative changes in pupillary unrest under ambient light in both suvorexant-treated and placebo-treated groups using a two-sided t-test.
Effect modification factors
Suvorexant effect modification by baseline cognitive status, insomnia symptoms, excessive sleepiness and sleep habits will be assessed by preoperatively administering the Montreal Cognitive Assessment (MOCA), Insomnia Severity Index, Epworth Sleepiness Scale (ESS) and the Athens Insomnia Scale (AIS), respectively (table 4). Study participants will also be offered a wrist actigraph to wear before surgery to quantitatively measure preoperative sleep duration and sleep habits. This will be done as an optional substudy and when feasible. Participants will be asked to wear the wrist actigraph for at least 3 nights so that circadian rhythms and average TST can be determined.45 Effect modification by these baseline factors will be assessed by comparing point estimate outcome effect sizes in subgroups (baseline cognitive impairment vs normal cognition, excessive sleepiness presence, insomnia symptom presence and preoperative short versus long sleep). These results will be considered hypothesis-generating for future study designs on postoperative sleep pharmacotherapy.
Summary of study questionnaires and assessments
Data management and monitoring
All participants will be assigned a subject study number so that their data will be de-identified. EEG data will be accessed through a secure cloud-based platform and backed up in secure local drive, while pupillometry and PVT data will be stored on a secure local drive. All other data will be stored on the secure web-based REDCap software. Technical appendix, statistical code and the relevant dataset will be available from the Vivli Clinical Research Data Repository (https://vivli.org/). This study will be monitored according to the Duke Clinical Quality Management Plan, which includes biannual independent reviews of all study-related documents and facilities. The principal investigator will also permit study-related monitoring, audits and inspections of all study-related documents and facilities by the IRB, sponsor, government regulatory bodies and university compliance and quality assurance groups.
Adverse events and safety
Adverse events will be assessed daily by study staff. The principal investigator will determine whether the adverse event was unrelated, unlikely related, possibly related, probably related or definitely related to study drug treatment. Any symptom, sign, illness or experience that develops or worsens in severity will be identified as an adverse event and reported to the sponsor and IRB, if necessary. All adverse events occurring during the study period will be recorded in the source document and case report form. The most common side effects of suvorexant include headache, diarrhoea, xerostomia, cough, abnormal dreams, dizziness, drowsiness and daytime tiredness. Minor side effects include sleep paralysis, sleepwalking, itchiness, nausea, vomiting, palpitations, daytime sedation and worsening of depression and suicidal ideation.46 47 Study drug-related adverse events will be reported for placebo versus suvorexant groups in the manuscript results. Study subjects may be withdrawn from the study at any time by the principal investigator.
In the case that three unexpected, related serious adverse events are reported in this study, an unblinded staff statistician will perform analyses to determine whether these related serious adverse events are associated with the suvorexant or placebo group. This information will then be used by a designated medical monitor, an appointed physician not involved in the study, to decide whether to continue the study.
Statistical analysis
Since some patients may miss a study drug dose on the first postoperative night, the primary analysis will be conducted on a modified intent-to-treat basis comparing TST on the first night that a patient received a study drug dose with a two-sample t-test. Secondary analysis will compare peak postoperative delirium severity scores between suvorexant and placebo groups with a two-sample t-test. This modified intent-to-treat analysis will only include those patients who received the study drug dose. Based on the American Academy of Sleep Medicine Insomnia Clinical Practice Guidelines, we will consider a 20 min difference in TST a clinically meaningful difference.48 We expect that a sample size of 130 subjects will yield a total of 116 subjects with complete primary and secondary endpoint data. Given an SD of approximately 35 min for TST in healthy adults, a sample size of 58 subjects per group will provide 86% power to detect a 20 min difference in TST between treatment groups using a two-sample t-test with a two-sided alpha of 0.05.31 Prrior to unblinding and analysis, additional patients may be recruited until a total of 116 subjects have complete primary and secondary endpoint data.
For the secondary endpoint, we will consider a 50% reduction in peak postoperative delirium severity scores to be a clinically meaningful difference. A 50% reduction in delirium severity is reasonable, as another study that included non-pharmacological sleep promotion found a reduction in delirium incidence by approximately 50%.49 Given a delirium severity SD of 1.8 and a mean peak 3D-CAM delirium severity score of 1.9 in the placebo group, a sample size of 58 patients per group will provide 80% power to detect a 0.95-point difference between treatment groups using a two-sided t-test with an alpha of 0.05. In order to control for preoperative delirium status, as well as other potential confounders, we will subsequently use multivariable linear regression to assess the presence of treatment effect. Delirium incidence and duration, two exploratory outcomes, will be assessed via logistic regression, time-to-event and zero-inflated log-linear modelling.
Exploratory endpoint analysis will compare the effects on subjective sleep quality, postoperative pupil diameter fluctuations, average response latency in psychomotor vigilance testing and total average electrographic sleep time over postoperative days 0, 1 and 2, if applicable, between suvorexant and placebo groups.
Precision variables include preoperative measures, including the score on the MOCA, excessive daytime sleepiness measured with the Epworth Sleepiness Scale, and poor preoperative sleep reported with the Athens Insomnia Scale.50–52
Ethics statements
Patient consent for publication
Acknowledgments
We thank all subjects who participate in the REPOSE study.
References
Footnotes
Contributors MD conceptualised the study. MD, JMF, MH, CEP, MCW, JMV, NMP, MEA, MS, WM, KR, MT and CS contributed to the study design. JMF, DT, SRW and MD contributed to drafting the test and preparing figures and tables. All authors critically reviewed and edited the final draft. MD is the guarantor.
Funding Supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, New Jersey, USA. The opinions expressed here are those of the authors and do not necessarily represent those of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, New Jersey, USA.
Competing interests Support for this study is provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co. (to MJD). MJD acknowledges additional support from NIA R01AG073598, K23AG084898, and P30AG072958 grants. MT acknowledges a clinical trial grant from Edwards Life Sciences Corporation.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.