Article Text
Abstract
Background Delayed graft function (DGF) continues to represent one of the most frequently encountered early complications following kidney transplantation. Despite notable progress in donor and recipient pretreatment protocols, diagnostic techniques and therapeutic approaches, the incidence of DGF, along with its associated short- and long-term sequelae, has not demonstrated a significant reduction. DGF is influenced by a multitude of factors, and individuals with exposure to these risk factors exhibit a markedly increased probability of developing DGF.
Objectives To systematically identify and evaluate risk factors associated with DGF in kidney transplant recipients.
Design A systematic review and meta-analysis
Data sources A comprehensive search was performed across multiple databases, including PubMed, Embase, The Cochrane Library, Web of Science, CNKI, Wanfang, VIP and SinoMed, from the inception of each database until 1 March 2024.
Primary outcome measures OR and OR 95% CI of risk factors for DGF.
Results The meta-analysis included 19 studies involving a total of 153 008 patients, of whom 96 596 (63.1%) developed DGF. The following risk factors for DGF were identified: prolonged cold ischaemia time (CIT) (OR=1.05, 95% CI=1.03 to 1.07, p<0.0001), elevated donor end-stage serum creatinine (OR=1.54, 95% CI=1.26 to 1.87, p<0.0001), extended dialysis vintage (OR=1.02, 95% CI=1.00 to 1.02, p=0.014), increased human leucocyte antigen (HLA) mismatch number (OR=1.19, 95% CI=1.06 to 1.33, p=0.004), higher donor body mass index (BMI) (OR=1.07, 95% CI=1.03 to 1.11, p<0.0001), advanced donor age (OR=1.02, 95% CI=1.01 to 1.03, p=0.003) and recipient diabetes mellitus (OR=1.52, 95% CI=1.40 to 1.64, p<0.0001).
Conclusion This meta-analysis identified seven significant risk factors for DGF, including prolonged CIT, elevated donor end-stage serum creatinine, extended dialysis vintage, increased HLA mismatch number, higher donor BMI, advanced donor age and recipient diabetes mellitus. These findings may offer potential insights for developing clinical strategies to mitigate the risk of DGF in kidney transplant recipients and improve postoperative management.
PROSPERO registration number CRD42024520542.
- Meta-Analysis
- Renal transplantation
- Risk Factors
- Systematic Review
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Literature included in multicentre Chinese and English core databases shows diverse data and a large sample size.
The exclusion of risk factors reported in fewer than three studies may have introduced selection bias, potentially omitting clinically relevant variables.
This study excluded material for which the entire text is not available for free.
Introduction
Delayed graft function (DGF) is one of the most common early complications following kidney transplantation. Current diagnostic criteria define DGF as the need for dialysis within the first seven postoperative days.1 The incidence of DGF has remained unchanged, and its short- and long-term consequences persist despite advancements in donor and recipient pretreatment, diagnostic techniques and therapeutic interventions.2 Studies related to this topic suggest that the incidence of DGF following deceased donor kidney transplantation ranges from 20% to 50%, while the incidence after living donor transplantation ranges from 4% to 10%.3 If DGF occurs without prompt intervention, it may lead to an increased risk of graft loss and reduced patient survival rates.4 Several studies5 have identified multiple factors associated with DGF development, and exposure to these factors appears to increase the likelihood of DGF occurrence. However, comprehensive systematic reviews and meta-analyses on DGF risk factors remain limited. This study aims to systematically review and analyse the risk factors for DGF, with the goal of providing references for clinical practice.
Materials and methods
This meta-analysis is registered with PROSPERO (registration number: CRD42024520542) and is conducted in compliance with the preferred reporting project6 for systematic evaluation and meta-analysis.
Search strategy
We conducted a comprehensive search of core databases, including the Chinese Knowledge Network (CNKI), the VIP database, the Wanfang database, the Chinese Biomedical Documentation Service (SinoMed), PubMed, Web of Science (WOS), Embase and the Cochrane Library, to identify studies related to DGF risk factors. The search period ranged from the establishment of each database to 31 January 2024. During the literature review process, we meticulously examined the exclusion criteria for study participants in each article to verify that the utilisation of organs obtained from executed prisoners was explicitly excluded. See the online supplemental file Search strategy for the search type of each database.
Supplemental material
English search terms: “kidney transplantation/ renal transplantation” “delayed graft function /DGF” “risk factors/factor/risk/predictive factor/influence factor/correlation”.
Taking PubMed as an example, the English retrieval terms were as follows: “delayed graft function” (Title/Abstract) OR “DGF” (Title/Abstract) AND “kidney transplantation” (MeSH) OR “renal transplantation” (Title/Abstract) OR “kidney transplant” (Title/Abstract) OR “renal transplant” (Title/Abstract) AND “risk factors” (MeSH) OR “factor” (Title/Abstract) OR “influence factor” (Title/Abstract) OR “predictive factor” (Title/Abstract) OR “correlation” (Title/Abstract).
Definition
DGF: DGF was defined as the requirement for at least one dialysis session within the first week following kidney transplantation.
Dialysis vintage: Dialysis vintage was defined as the period from the initiation of the initial dialysis session prior to transplantation until the time of kidney transplantation.
Inclusion and exclusion criteria
Inclusion criteria
Study objects: adult recipients after renal transplantation.
Study type: case-controlled study and cohort study.
DGF definition: considering the impact of DGF on morbidity, the definition of DGF was limited to the requirement for at least one dialysis session within the first week following kidney transplantation.
Have reasonable statistical indicators.
Languages in Chinese and English.
Exclusion criteria
Patients who had non-single renal transplants, such as pancreatic joint transplants.
Unable to access the full literature free of charge.
Low-quality literature on the Newcastle-Ottawa Scale (NOS).
Dissertation.
Assessment of risk of bias
The methodological quality of the included studies was evaluated using the NOS, a widely recognised quality-assessment tool for case-control and cohort studies. The NOS assesses studies across three domains, comprising eight items, which include population selection, comparability and exposure/outcome evaluation. The scale employs a semiquantitative star-based scoring system, with a maximum of two stars allocated for comparability and one star for each of the remaining items, yielding a total possible score of nine stars. A higher NOS score generally indicates superior methodological quality and a lower risk of bias in the study.
Data extraction
Two researchers independently reviewed, extracted and confirmed the literature. If there is a dispute, the two researchers will consult and debate it, or a third researcher will decide. Two researchers independently examined the themes and summaries for the first screening, then read the complete text to decide the study’s inclusion based on the integration and exclusion criteria.
The information extracts include:
The basic characteristics of the study: subject, author, year of publication, type of study.
The basic features of the subject: total number of cases, DGF number, non-DGF case number, DGF diagnostic criteria.
Outcome-related indicators: incidence rate of DGF, risk factor of DGF, OR and its 95% CI confidence range.
Statistical methods
The retrieved documents were imported into EndNote and independently filtered by two researchers. After the final filtering of the documents, the relevant data from the preliminary documents were transferred to Excel. Assessment of risk of bias uses the NOS to evaluate cohort studies or case-control studies.7 Use Stata V.15.0 for meta-analysis, import the data from the Excel table into the Stata software, and merge OR values, lower CI and upper CI, respectively. The inclusion of literature heterogeneity test using the Q test and I2 test, if p>0.10, I2≤50%, is considered to include lower heterogeneity between studies, selecting the fixed effect model; if p≤0.10 and I2>50%, this is considered to be high heterogeneity between the studies, the use of the random effect model for data combination, using sensitivity analysis remove the literature method one by one to find heterogeneity and explore the stability of the results. Explore publication bias using the Egger test method, use funnel plot and Egger testing to assess whether there is bias in the meta-analysis results, p<0.05 for differences are statistically significant.
Patient and public involvement
None.
Results
Study selection and study characteristics
According to the search strategy, a total of 3343 literatures were retrieved, of which 118 were Chinese and 3225 were English, and a total of 2103 were obtained after removal of multiple references; 1968 were screened after reading titles and abstracts; 135 were obtained through preliminary screening; and 19 literatures were finally included after careful reading and analysis. The 20 articles excluded under the definition are as follows: The majority of these 20 articles were prior years’ Chinese publications, and they did not provide a definition of DGF. Second, the definitions of DGF in the remaining articles are not particularly uniform, such as ‘oliguria or anuria after surgery, at least 1 dialysis treatment within 1 week, serum creatinine value>400 µmol/L on the 7th day after surgery’ (n=2), ‘serum creatinine decreased by<10% per day for 3 consecutive days, dialysis is required in the first week of kidney transplantation’ (n=1), ‘serum creatinine level>3 mg/dL on the fifth day of renal transplantation’ (n=1), ‘daily serum creatinine decrease<10% for three consecutive days, requiring dialysis during week 1 of renal transplantation, or serum creatinine level>3 mg/dL on day 5 of renal transplantation’ (n=1), ‘Serum creatinine level>400 lmol/L 7 days after transplantation and/or hemodialysis required during the first week after transplantation’ (n=1), ‘serum creatinine level increased, remained unchanged, or immediately decreased by less than 10% per day during the first.’ 17 of the 19 literatures featured were in English, with 2 in Chinese. 16 of the studies were case-control, while 3 were cohort studies. This study includes 153 008 participants and 36 risk variables. It is important to note that none of the themes discussed in this study used organs from executed prisoners. Figure 1 depicts the literature screening procedure, whereas table 1 describes the literature features.
Study characteristics
Flow diagram of study selection. DGF, delayed graft function.
Literature quality evaluation
The NOS scores of 16 case-control studies were all≥7 points, with 5 at 7 points, 6 at 8 points and 5 at 9 points. Three cohort studies used the NOS score, with two scores of 8 and one of 7. The overall quality of the literature was high, with score specifics provided in online supplemental tables 1 and 2.
Supplemental material
Result of meta-analysis
We compiled 36 factors in 19 articles, 11 of which were mentioned in cold ischaemia time (CIT), followed by end-stage serum creatinine of the donor (seven times) and dialysis vintage (five times). A total of 8 factors were mentioned≥3 times, while the other 24 factors were not included in the meta-analysis due to the small number of articles mentioned. Table 2 summarises the key risk factor analysis results.
Summary of analysis results of main risk factors
Cold ischaemia time
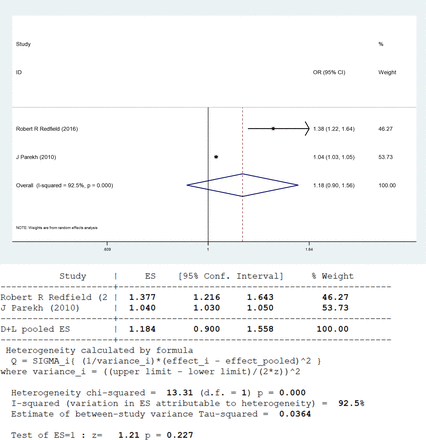
CIT was cited as a risk factor for DGF in 11 papers, with a total of 59 089 renal transplant recipients, 16 624 of whom acquired DGF. Figure 2 shows that the CIT was significantly longer in the DGF group compared with the non-DGF group, with a combined effect value of OR=1.05, OR 95% CI=1.03 to 1.07, Z=5.36, p<0.0001 and significant heterogeneity (I2=90.5%, p<0.0001). However, living patients who had kidney transplantation were included as research subjects in three publications. The nine literatures on dead donors exhibited a cumulative effect value of OR=1.04, with an OR 95% CI of 1.03 to 1.06, Z=4.58 and p<0.0001. There was significant heterogeneity (I²=90.6%, p<0.0001), although the outcome was not significantly different from the 11 included publications. In the two studies on living donors, the overall effect value was 1.18, with an OR 95% CI of 0.90 to 1.56, Z=1.21 and p=0.227. The study found high heterogeneity (I²=92.5%, p<0.0001), which contradicted the findings of the 11 included papers (see figures 3–4). We performed a sensitivity analysis, and the findings revealed that the results of 11 studies were stable, as shown in figure 5.
Meta-analysis of cold ischaemia time from all the references cited. ES, effect size.
Meta-analysis of cold ischaemia time from deceased donor. ES, effect size.
Meta-analysis of cold ischaemia time from living donor. ES, effect size.
Sensitivity analysis of cold ischaemia time.
End-stage serum creatinine of the donor
A total of seven studies identified end-stage serum creatinine in the donor as a risk factor for DGF, involving 29 960 renal transplant recipients and 9144 occurrences of DGF. The combination effect value of OR=1.54, OR 95% CI=1.26 to 1.87, Z=4.28, p<0.0001 and heterogeneity significant (I2=94.8%, p<0.001) indicates that end-stage serum creatinine of the DGF group is significantly greater than that of the non-DGF group. Results of the sensitivity analysis show that the results of seven studies were stable (see detailed figure 6 and online supplemental figure 1).
Supplemental material
Meta-analysis result of end-stage serum creatinine of the donor. ES, effect size.
Dialysis vintage
The study found a combined effect of OR=1.02, OR 95% CI=1.00 to 1.04, Z=2.46, p=0.014. The DGF group had a considerably longer dialysis vintage than the non-DGF group; however, there was significant heterogeneity (I2=98.6%, p<0.0001). However, one article’s study subjects included living patients who had kidney transplantation, and the overall impact value for the three papers on deceased donors was OR=1.01. The OR 95% CI=1.00 to 1.01, Z=2.05, p=0.041 and significant heterogeneity (I2=94.2%, p<0.0001) resulted in little difference from the results of the inclusion of four articles, as shown in online supplemental figures 2 and 3. The sensitivity analysis yielded steady results, as shown in detail in online supplemental figure 4.
Supplemental material
Supplemental material
Supplemental material
HLA mismatch number
A total of four articles alluded to the human leucocyte antigen (HLA) erroneous allocation as a risk factor for the development of DGF, with a total of 28 720 cases of cases of kidney transplant recipients and 9023 DGF occurrences. The combined effect value OR=1.19 (95% CI=1.06 to 1.33, Z=2.91, p=0.004) suggests that the HLA error allocation is considerably bigger than the non-DGF group, although the result has a stronger heterogeneity (I2=87%, p<0.0001). However, the study subjects for two articles included living patients who had kidney transplantation. The two papers on deceased donors had a total impact value of OR=1.97. The meta-analysis revealed a pooled OR of 0.54 to 7.20 (95% CI) with a Z-score of 1.03 (p=0.304), indicating no statistical significance. Substantial heterogeneity was observed (I²=92.8%, p<0.0001). Notably, these findings were inconsistent with the results reported in the four included studies. Regarding the subgroup analysis of living donors (n=2 studies), the pooled OR was 1.19 (95% CI: 1.04 to 1.38; Z=2.45, p=0.014), demonstrating statistical significance. Moderate heterogeneity was present in this subgroup (I²=85.0%, p=0.010), with results showing consistency with the four included studies (see online supplemental figures 5–7). Sensitivity analysis confirmed the robustness of these findings, as presented in online supplemental figure 8.
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Recipient body mass index
Three studies investigated the association between recipient body mass index (BMI) and DGF risk, encompassing a total cohort of 25 060 kidney transplant recipients, including 6472 DGF cases. Random-effects model analysis yielded a pooled OR of 1.23 (95% CI: 0.99 to 1.53; Z=1.85, p=0.065), indicating no statistically significant difference in recipient BMI between DGF and non-DGF groups. However, substantial heterogeneity was observed (I²=90.4%, p<0.0001). Notably, one study exclusively focused on living donor transplantation, while the remaining two studies examined deceased donor transplantation, showing a pooled OR of 1.91 (95% CI: 0.85 to 1.66; Z=1.03, p=0.303) with moderate heterogeneity (I²=75.8%, p=0.042). The exclusion of the living donor study demonstrated minimal impact on overall effect estimates, as illustrated in online supplemental figures 9 and 10. Sensitivity analysis revealed significant methodological differences in Irish’s study compared with other investigations, as detailed in online supplemental figure 11.
Supplemental material
Supplemental material
Supplemental material
Donor BMI
Three studies identified donor BMI as a significant risk factor for DGF, encompassing a total of 3832 renal transplant recipients and 203 cases of DGF. The pooled effect size, expressed as an OR, was 1.07 (95% CI: 1.03 to 1.11; Z=3.74, p<0.0001), indicating a statistically significant association between donor BMI and DGF. Heterogeneity among the studies was low (I²=25.2%, p=0.263). However, one of the included studies focused on living kidney transplant recipients, while the remaining two studies examined deceased donors. The pooled OR for the two studies involving deceased donors was 1.06 (95% CI: 1.02 to 1.10; Z=3.03, p=0.002), with no observed heterogeneity (I²=0.0%, p<0.0001). These findings were consistent with the results obtained from the analysis of all three studies (see online supplemental figures 12 and 13). Sensitivity analysis further confirmed the stability of the results across the three studies (see online supplemental figure 14).
Supplemental material
Supplemental material
Supplemental material
Donor age
Four studies identified donor age as a risk factor for DGF, encompassing 28 936 renal transplant recipients and 8708 DGF cases. The pooled analysis demonstrated a significant association between donor age and DGF (OR=1.02, 95% CI=1.01 to 1.03, Z=2.96, p=0.003). However, substantial heterogeneity was observed among studies (I2=84.6%, p<0.0001). Notably, one study included living donor transplant recipients, while the remaining three studies focused on deceased donor transplants. The analysis restricted to deceased donor studies yielded similar results (OR=1.02, 95% CI=1.01 to 1.03, Z=4.03, p<0.0001), with reduced but still considerable heterogeneity (I2=74.6%, p=0.020). These findings are presented in online supplemental figures 15 and 16. Sensitivity analysis revealed variability in effect estimates across studies, as illustrated in online supplemental figure 17.
Supplemental material
Supplemental material
Supplemental material
Recipient diabetes
Four studies identified recipient diabetes as a risk factor for DGF, encompassing a total of 28 951 renal transplant recipients, among whom 2223 experienced DGF. The pooled effect size (OR=1.52, 95% CI=1.40 to 1.64, Z=10.30, p<0.0001) suggested a significant association between recipient diabetes and DGF, with low heterogeneity observed (I²=39.3%, p=0.176). The results of these four studies demonstrated relative stability. Notably, two of the included studies focused on living donor kidney transplantation. For deceased donor transplantation, the pooled effect size was OR=1.36 (95% CI=1.12 to 1.66, Z=3.13, p=0.002), with low heterogeneity (I²=43.7%, p=0.183). This result did not significantly differ from the overall findings of the four studies. Regarding living donor transplantation, the pooled effect size was OR=1.55 (95% CI=1.42 to 1.69, Z=9.89, p<0.0001), also exhibiting low heterogeneity (I²=42.4%, p=0.188). These results were consistent with the overall findings, as detailed in online supplemental figures 18–20. Sensitivity analysis further confirmed the stability of the results across the four studies, as illustrated in online supplemental figure 21.
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Publication bias analysis
The assessment of potential publication bias was conducted using different statistical methods based on the frequency of references for each factor. For CIT, loop plots were employed due to the availability of>10 reference studies, while Egger’s test was applied to dialysis vintage and donor’s end-stage serum creatinine. The loop plot analysis revealed that most CIT studies were clustered around the midline, although with asymmetric distribution. Egger’s test results indicated no significant publication bias for any of the factors (CIT: p=0.075; dialysis vintage: p=0.089; donor’s end-stage serum creatinine: p=0.362), with all p values exceeding the 0.05 threshold. Detailed results are presented in online supplemental figure 22.
Supplemental material
Discussion
To the best of our knowledge, this represents the first meta-analysis to systematically evaluate the risk factors associated with DGF, offering a significant contribution to clinical practice. DGF, a common complication in the early post-transplant period, has attracted considerable attention in the research community. Numerous studies investigating the pathogenesis of DGF8 have identified ischaemia-reperfusion injury as a primary mechanism, with the duration of cold and warm ischaemia being critical factors. Our meta-analysis revealed that CIT in both all donors and deceased donors exhibited statistically significant differences between DGF and non-DGF groups, with longer CIT correlating with a higher incidence of DGF. However, no significant association was observed in living donors, potentially due to the limited number of relevant studies (only two). Notably, the analysis demonstrated substantial heterogeneity, primarily attributable to transplantation type and sample size. Heterogeneity was reduced after excluding studies involving living kidney transplantation. Among deceased donor studies, heterogeneity was observed between large-sample and small-sample studies. Furthermore, our prior research9 suggested that CIT may not be an independent risk factor for DGF, possibly due to variations in DGF definitions and kidney transplantation types. Warm ischaemia time was not included in the analysis due to insufficient data in the reviewed literature. The meta-analysis results for CIT exhibited significant heterogeneity. Recent advancements in ischaemia-reperfusion injury prevention have shown promising results, with studies demonstrating the potential benefits of machine perfusion1 10 and antioxidants8 in mitigating ischaemia-reperfusion injury. These technological developments hold promise for improving outcomes in organ transplantation. However, further experimental validation is necessary before these methods can be widely implemented in clinical practice.
Previous studies11 12 have demonstrated that elevated end-stage creatinine levels are significantly associated with an increased risk of DGF, with a positive correlation observed between creatinine levels and DGF risk. This finding is consistent with our analytical results. However, significant heterogeneity was observed in the analysis, primarily attributable to variations in sample sizes across studies, with notable differences between studies with larger versus smaller sample sizes. The definition of DGF in the literature13 14 extends beyond postoperative dialysis requirements to include postoperative creatinine values as diagnostic criteria. According to Sun et al,15 donor age, recipient obesity and recipient hypertension are significant factors contributing to elevated postoperative creatinine levels in recipients. Therefore, to optimise post-transplant outcomes and enhance graft survival, it is recommended to implement rigorous preoperative assessments for elderly donors and improve recipients’ postoperative condition.
Accumulated evidence suggests that prolonged preoperative dialysis duration adversely impacts renal allograft survival and is associated with an elevated risk of DGF,16 a finding corroborated by our meta-analysis. However, significant heterogeneity was observed across studies (I²=75%). Subgroup analyses identified transplant type (living vs deceased donor) and publication year as primary sources of heterogeneity. Exclusion of studies involving living donor kidney transplants partially mitigated heterogeneity (I²=52%). Among deceased donor recipient studies, sensitivity analysis revealed that the 2013 cohort contributed disproportionately to heterogeneity compared with cohorts published in 2020–2021, potentially reflecting era-specific differences in clinical practice.
Mechanistically, extended dialysis vintage may potentiate cardiovascular comorbidities, including left ventricular dysfunction and hypertrophy, likely mediated by dyslipidaemia-driven acceleration of atherosclerosis. Furthermore, prolonged dialysis exposure correlates with suboptimal HLA matching and elevated panel reactive antibody (PRA) positivity,17 18 both established immunologic risk factors for graft injury. Although preliminary evidence suggests a lower incidence of DGF in pretransplant peritoneal dialysis recipients compared with haemodialysis patients,19 the limited number of studies addressing dialysis modality precluded an analysis in this work.
The HLA, encoded by the major histocompatibility complex genes, plays a crucial role in transplantation immunology. Among the HLA antigens, HLA-A, B and DR are particularly relevant to transplantation outcomes, with HLA-DR and HLA-B matching demonstrating significant importance in renal transplantation.20 Current clinical practice uses HLA mismatch grading to assess donor–recipient histocompatibility. A complete HLA mismatch in allograft transplantation may involve up to 14 incompatible HLA molecules, which are introduced into the recipient’s system during transplantation. Clinical evidence consistently demonstrates an inverse correlation between the degree of phenotypic mismatch and graft survival rates. Zhao et al21 reported a significant increase in DGF incidence when HLA mismatches exceeded two, a finding corroborated by our meta-analysis results identifying HLA mismatch as a significant risk factor for DGF. However, subgroup analysis of deceased donor transplants revealed no statistically significant association, potentially attributable to variability in HLA mismatch classification across studies. Among the four included studies, Redfield et al22 identified a significant increase in DGF incidence with HLA mismatches exceeding four, while Chen et al23 demonstrated HLA-class I mismatch as an independent risk factor for DGF. The remaining two studies24 25 reported a dose-dependent relationship between increasing HLA mismatch and DGF incidence. Notably, our analysis revealed substantial heterogeneity, primarily attributable to variations in transplant type and sample size. Exclusion of living donor transplantation studies resulted in a measurable reduction in heterogeneity.
Numerous studies have identified recipient BMI as a significant risk factor for DGF, with Jindal et al demonstrating a positive correlation between recipient obesity and increased DGF incidence.26 In the present analysis, recipient BMI was consistently treated as a continuous variable across all included studies, revealing a dose-dependent relationship between elevated BMI and higher DGF rates. This association may be attributed to several pathophysiological mechanisms, including compromised vascular access, prolonged CIT and extended anastomosis duration in obese recipients.27 Furthermore, obesity-related deficiency in anti-inflammatory factors may exacerbate ischaemia-reperfusion injury in transplanted kidneys. However, our meta-analysis revealed no statistically significant difference in BMI between DGF and non-DGF groups, with substantial heterogeneity observed across studies. Analysis identified transplantation type and sample size as primary sources of heterogeneity. Exclusion of living donor transplantation studies resulted in moderate reduction of heterogeneity. Current clinical guidelines28 recommend maintaining recipient BMI below 25 kg/m², with mandatory patient education regarding obesity-associated complications and perioperative risks for candidates exceeding this threshold. For transplant candidates with BMI>25 kg/m², implementation of weight management strategies is strongly advised. Bariatric surgery may be considered for selected patients, though its potential impact on transplantation outcomes requires careful consideration. While bariatric procedures do not significantly affect graft prognosis, they are associated with a 30% increased risk of acute rejection episodes.29
Elevated BMI in donors has been associated with comorbidities such as hypertension and hyperlipidaemia, which may compromise the quality of donor kidneys, potentially increasing the risk of DGF and adversely affecting graft survival rates.30 Additionally, post-transplantation, obese donors often exhibit an increased glomerular filtration rate, which can lead to tubular dilatation and subsequent complications, including hypertension, hyperlipidaemia and microproteinuria.31 In this study, a statistically significant difference in donor BMI was observed between the DGF and non-DGF groups, with low heterogeneity. However, conflicting evidence exists, as some studies32 have reported no direct correlation between donor or recipient BMI and postoperative renal function recovery. Current guidelines recommend avoiding the use of donor kidneys from individuals with a BMI>35 kg/m². For donors with a BMI>30 kg/m², transplantation should be considered only after thorough evaluation and when no alternative donors are available. In such cases, preoperative weight loss should be encouraged, and the risks and benefits must be carefully communicated to the donor.33
The proportion of elderly individuals receiving kidney transplants has increased in parallel with population ageing trends. In several European countries, donors aged over 70 years account for approximately 20% of kidney transplant recipients.34 In this analysis, donor age was analysed as a continuous variable, demonstrating a positive correlation with DGF incidence. Substantial heterogeneity was observed in the initial analysis, primarily attributable to transplantation type and sample size variations. Exclusion of living donor transplantation studies resulted in measurable reduction of heterogeneity. The scientific community has shown increasing interest in this research area, particularly regarding age-related physiological changes. Renal allografts from older donors demonstrate increased susceptibility to ischaemia-reperfusion injury, potentially due to reduced renal functional reserve compared with younger donors.35 However, conflicting evidence exists in the literature. While Jeong Hoon Lim et al36 reported comparable outcomes in DGF incidence, graft survival, and acute rejection-free survival between elderly (>60 years) and younger recipients, our analysis identified advanced donor age as a significant risk factor for DGF development. Benoit Mesnard et al37 conducted a meta-analysis supporting the reliability of elderly donors (≥70 years) as graft sources. Furthermore, Chavalitdhamrong et al38 demonstrated that satisfactory transplant outcomes can be achieved with donors over 70 years when comprehensive evaluation and matching protocols are implemented.
Post-transplant pharmacological regimens involving corticosteroids (eg, methylprednisolone, prednisone) and calcineurin inhibitors are recognised contributors to secondary hyperglycaemia,1 which may impair glycaemic regulation and potentially accelerate renal allograft dysfunction through diabetic nephropathy mechanisms.2 Pharmacokinetic variability exacerbated by glycaemic fluctuations may amplify nephrotoxic effects.39 Clinical evidence demonstrates that post-transplant diabetes mellitus (PTDM) is associated with elevated risks of cardiovascular complications, infectious morbidities and unfavourable allograft outcomes.40 Accumulating data from observational studies41 42 collectively demonstrate that both pre-existing diabetes and PTDM correlate with increased incidence of DGF and reduced graft survival rates. Our meta-analysis corroborates these findings, identifying diabetes status as a significant DGF risk factor (I²=23%, indicating low heterogeneity). These observations underscore the necessity for comprehensive pretransplant metabolic profiling, including evaluation of obesity indices, lipid parameters and familial diabetes predisposition—established predictors of PTDM development.43 Preoperative metabolic optimisation strategies, validated in general populations for type 2 diabetes prevention,44 warrant particular consideration in transplant candidates. Given the diabetogenic potential of maintenance immunosuppression,45 protocol-driven selection of metabolic-friendly regimens (eg, corticosteroid minimisation, mTOR inhibitor avoidance) is recommended for high-risk recipients.46
Growing evidence suggests that genetic susceptibility may contribute to the pathogenesis of DGF, particularly through polymorphisms affecting oxidative stress pathways and immune regulation, highlighting the potential utility of preoperative genetic assessment in high-risk transplant recipients. Specific single nucleotide polymorphisms have been associated with increased renal susceptibility to ischaemia-reperfusion injury and poorer post-transplant outcomes.47 These polymorphisms can be broadly categorised into five functional groups48: (1) oxidative stress-related variants (MnSOD,49 rs1001179 (−262 C/T)50 and NADPH oxidase p22phox C242T51); (2) telomere maintenance alterations (hTERT, BICD1 and chromosome 18 polymorphisms52); (3) chemokine signalling modifications (IL-1 receptor antagonist,53 TNF-α54 and CX3CR1 V249I55); (4) T-cell homeostasis regulators (CTLA-456); and (5) immune response modulators (toll-like receptor system,57 epoxyeicosatrienoic acids58 and nitric oxide synthase variants59). The identified polymorphisms have been implicated in the pathogenesis of DGF through distinct molecular mechanisms. Preoperative genetic screening of recipients and potential living donors may help stratify DGF risk, though clinical validation of these markers remains ongoing. Patients should receive counselling regarding genetic risk factors and preventive strategies. Furthermore, surgical protocols should emphasise optimisation of donor kidney preservation times (both warm and cold ischaemia), while post-transplant management requires individualised immunosuppressive regimens based on recipient genetic profiles.
Limitations
We must acknowledge that this analysis has some limitations: first, we chose a number of larger and representative databases with data from many countries; however, there are differences in medical technology between regions that cannot be well harmonised, which is also one of the reasons for heterogeneity. Second, because the impact of the DGF definition on sickness incidence will yield various analytical outcomes, we have restricted the DGF definition. Furthermore, we omitted articles for which the complete text is not freely available, potentially ignoring other high-quality work. Furthermore, the period frame from which we obtained data is from the beginning to the present, and given the rapid advancement of medical technology, this is a diverse source. We can only evaluate risk factors in studies with a sample size of≥3 due to the unpredictability of study outcomes and limited assertions. Finally, this study explored the risk factors for DGF, but it was not possible to calculate the risk threshold for each factor because, in the included articles, apart from categorical variables, other variables were analysed as continuous variables in the original literature, meaning that as each unit of the variable increases, the risk of DGF also continuously increases.
Conclusions
In conclusion, this meta-analysis has identified key risk factors for DGF, offering valuable insights for clinical decision-making in donor selection, preoperative preparation and postoperative management to enhance renal function recovery and improve patient outcomes.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
Acknowledgments
The authors extend their sincere gratitude to all individuals who provided invaluable support throughout the course of this study. Special thanks are extended to ZL for her instrumental guidance in manuscript preparation and topic selection. Her in-depth knowledge and constructive feedback significantly contributed to the successful completion of this article, enabling the authors to refine their ideas and present a more comprehensive analysis. The authors also wish to acknowledge the contributions of Director Ma Fang. The systematic assessment and meta-analysis course offered by Director Ma provided a solid theoretical foundation for this research. The concepts and methods learned from the course served as a crucial compass, guiding the authors in their data analysis and interpretation, and ensuring the scientific rigour of the study. Finally, MK's contributions are highly appreciated. MK actively participated in the drafting of the manuscript, collaborated closely with the authors in data extraction and literature screening and offered abundant academic advice during the research process. Her efforts not only enhanced the quality of the study but also broadened the authors’ perspectives, leading to a more in-depth exploration of the research topic. Without the support and contributions of these individuals, this study would not have been possible. Their assistance has been an essential part of this research journey, and the authors are deeply indebted to them
References
Footnotes
Contributors ZY and MK were responsible for literature screening, quality evaluation and data extraction; ZY completed meta-analysis; ZL was mainly responsible for negotiation and resolution of differences, as well as guidance of manuscripts. All authors contributed to the writing of the final manuscript. The guarantor of this paper is ZL.
Funding This work was supported by (Yunnan Provincial Department of Science and Technology Kunming Medical University Applied Basic Research Joint Special Fund Project) grant number (202201AY070001-080), (Cultivating Plan Program for the Leader in Science and Technology of Yunnan Province) grant numbers (202305AD160007), (Innovation Fund project for postgraduate students in 2024) grant number (2024S217), (Yunnan health training project of high level talents) grant number (D-2024022).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.