Article Text
Abstract
Objective We assessed whether Penthrox (methoxyflurane) can provide better pain relief for elective, outpatient interventional procedures compared with other methods of pain control.
Design Systematic review.
Data sources Cochrane Central Register of Controlled Trial, MEDLINE, Emcare, Embase, ClinicalTrials.gov and the Cumulative Index to Nursing and Allied Health Literature were searched from inception to 6 November 2023.
Eligibility criteria All published studies including randomised controlled trials (RCTs), cohort studies and case–control studies investigating Penthrox for pain relief, during and/or after an interventional procedure, were included.
Data extraction and synthesis Two independent reviewers identified eligible studies and retrieved data. Risk of bias was assessed using Cochrane Collaboration Risk of Bias V.2.0 and Risk of Bias in Non-randomised Studies of Interventions (ROBINS I) tools. A meta-analysis was not possible due to heterogeneity.
Results The literature search yielded 1189 records. 12 studies were eligible for inclusion: 5 RCTs and seven non-randomised studies of interventions (NRSIs). Penthrox was used for a range of procedures across five medical specialities: gastroenterology, gynaecology, haematology, orthopaedics and urology. Two RCTs showed a significant reduction in intraprocedural pain when Penthrox was compared with placebo. However, three RCTs where Penthrox was compared with either placebo or intravenous sedation showed no significant differences in pain or comfort level. Similarly, the NRSIs showed variable results. Most patients were satisfied (63% to >98%; seven studies) with Penthrox, and seven out of eight studies reported that the majority of participants would use it again (46.8%–95%; eight studies). No serious adverse events were reported.
Conclusion Penthrox (methoxyflurane) shows promise as an analgesic for invasive, elective interventional procedures in the outpatient setting, although the relative benefits appear to vary depending on intervention and comparator pain control measures. There is a pressing need for robustly conducted, large, generalisable multicentre RCTs to evaluate the effectiveness of Penthrox.
PROSPERO registration number CRD42023404574.
- Drug Therapy
- Endoscopy
- GYNAECOLOGY
- UROLOGY
- HAEMATOLOGY
- PAIN MANAGEMENT
Data availability statement
Data are available on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
A clinically focused question was investigated using the population, intervention, comparator and outcome model.
A thorough search strategy was employed with multiple databases.
No language restrictions and broad search terms reduced selection bias.
Included non-randomised, interventional studies to maximise available data.
Heterogeneity and methodological limitations prevented a meta-analysis.
Introduction
Methoxyflurane (2,2-dichloro-1,1-difluoro-1-methoxyenthane) is a volatile fluorinated hydrocarbon, first introduced as an inhalational anaesthetic for human use after its original clinical trial in 1960.1 2 Its use in anaesthetics was discontinued by the late 1970s after some reports of dose-related nephrotoxicity emerged and with the introduction of newer volatile anaesthetic agents.3 4
Since then, methoxyflurane has regained popularity and has been repurposed for use at lower doses as an emergency analgesic in adult trauma patients.5–7 Low-dose methoxyflurane can be self-administered using a portable, disposable single-use handheld inhaler (Penthrox), also colloquially known as the ‘Green Whistle’. Penthrox is currently the only available medicinal form of methoxyflurane and contains 3 mL of inhalational vapour with 99.9% methoxyflurane and 0.01% butylated hydroxytoluene.8 The maximum methoxyflurane dose to produce analgesia is limited to 6 mL/day, which is well below the reported level of risk of nephrotoxicity in the literature.9 Penthrox has now been approved in over 55 countries including Canada, Europe, the UK, Middle East, Africa, Australia and some states within the USA, with no published reports of serious side effects to date.10
The effectiveness, safety and simplicity of Penthrox in acute trauma settings have led to it being used during planned, invasive ambulatory or outpatient procedures across a number of medical specialities. Penthrox may have properties that make it superior to other commonly used methods of pain control including analgesics like paracetamol, non-steroidal anti-inflammatory drugs (NSAIDs), opioids and nitrous oxide (Entonox), local anaesthesia and procedural sedation. These properties include a stronger analgesic effect, reduction in anxiety, no significant impact on cardiorespiratory function and a short half-life such that a formal recovery process is not required following its use.10 11
Since the use of Penthrox as a self-administered, portable single-use handheld inhaler outside of the acute trauma setting is in its infancy, current data on its utility in elective, interventional procedures are limited to small observational cohorts and randomised controlled trials (RCTs). Technological advances have allowed more planned invasive procedures to be undertaken in conscious patients outside of the operating theatre setting. In light of this contemporary medical practice and the increasing use of Penthrox, there is an urgent need to evaluate the evidence regarding its role in improving the experience of patients undergoing invasive outpatient or ambulatory procedures so that it is not introduced indiscriminately into NHS practice without evidence of benefit, harm and resource use. This will also allow for the identification of areas where further primary research is required. We therefore performed a systematic review investigating the efficacy, safety, feasibility and acceptability of Penthrox as pain relief when used for elective and invasive interventional procedures in the outpatient or ambulatory setting.
Methods
Protocol and guidance
The study protocol was registered on the PROSPERO International Prospective Register of Systematic Reviews (CRD42023404574),12 and the study was designed using guidance from the Cochrane Handbook for Systematic Reviews of Interventions 13 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (online supplemental figure 1).14
Supplemental material
Inclusion criteria
All published studies investigating the use of methoxyflurane in the form of Penthrox for pain relief during and/or after an elective invasive procedure in the ambulatory or outpatient setting were included for review. All RCTs, cohort studies and case–control studies were included. Case series and conference abstracts with sufficient data and information on methodology to assess were also included due to the paucity of available data. Language restrictions were not applied.
Exclusion criteria
Case reports were excluded. Studies involving the use of methoxyflurane in any other concentration than that in Penthrox were excluded. Studies evaluating emergency or semielective procedures or that involved patients already likely to be in pain (eg, changing of burn dressings) were excluded. Studies not reporting on the primary or secondary outcomes listed below were excluded. The authors of any study with insufficient information available to make an adequate assessment were contacted, where possible, and these were then excluded if sufficient data and methodology were not available.
Outcomes
The primary outcome was the effect of Penthrox on pain either during or after the procedure. Secondary outcomes included the impact of Penthrox on anxiety, adverse events, patient satisfaction/acceptability, clinician satisfaction/acceptability and clinician rating of patient cooperation.
Search strategy
The Cochrane Central Register of Controlled Trials, MEDLINE, Emcare, Embase, ClinicalTrials.gov and the Cumulative Index to Nursing and Allied Health Literature were searched from inception to the 6 November 2023 (online supplemental documents 1 and 2). A combination of the keywords “methoxyflurane”, “outpatient”, “ambulatory”, “scop*”, “surgery”, “biopsy”, “tooth extraction” and “cataract” and their associated medical subject headings were used to search the aforementioned databases, without any filters or limits. The reference lists of the included studies were reviewed, and any additional studies that had not been identified by our systematic review were included if deemed suitable according to our eligibility criteria.
Supplemental material
Supplemental material
Study selection
Duplicates were identified and removed by the primary reviewer (BZFS). Two reviewers (BZFS and PMDS) independently screened all titles and abstracts of the included articles. The full-text articles (where available) of potentially eligible studies were then retrieved for review and screened. Any disagreement was resolved through discussion, or if required, by an independent third reviewer (PS).
Data collection
Data from the included studies were independently extracted by two reviewers (BZFS. and PMDS) and collated onto a standardised data extraction form on Microsoft Excel. Any disagreement was resolved through discussion, or if required, by an independent third reviewer (PS). In addition to the clinical outcomes, data were collected regarding the study type, patient demographics and exclusion criteria for the use of Penthrox.
Assessment of risk of bias
The risk of bias assessment was independently carried out by the same two reviewers (BZFS and PMDS) with any disagreement resolved through consensus or by a third reviewer (PS). Assessments for RCTs were carried out using the Cochrane Collaboration Risk of Bias V.2.0 tool and for observational studies using the Risk of Bias in Non-randomised Studies of Interventions (ROBINS I) tool.15 16 The authors of included studies that had insufficient information regarding their methodology were emailed for further details.
Data synthesis
The study designs, methodology, procedures and outcome assessment of the included studies were too heterogeneous to allow a meta-analysis to be performed, and so a narrative, qualitative review was performed instead as a more appropriate way of synthesising and presenting the available evidence. The raw dataset from one study was publicly available on the internet and analysed to obtain relevant information for the purpose of the systematic review.17
Results
Study selection
The study selection process is summarised in figure 1. The literature search yielded 1432 records with 246 records identified as duplicates. Three studies were identified through other sources giving a total of 1189 titles and abstracts that required screening, leaving 34 full-text studies which were assessed for eligibility, of which 12 were included in the narrative review. The reference lists of the included articles were scanned to ensure no other relevant papers were overlooked.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram depicting the study selection process.
Study characteristics
Tables 1 and 2 outline the study characteristics of the included studies and detail the study type, age, population characteristics, types of procedure performed and pain relief administered. Of the 12 included studies, 5 were RCTs (table 1)17–21 and 7 (table 2) were cohort studies (of which, 5 were prospective22–26 and 2 included a combination of prospective and retrospective data27 28). One study was initially presented as a conference abstract,17 and the full-text article was published at a later date.
Summary of study characteristics (RCTs)
Summary of study characteristics (non-randomised studies of interventions)
The included studies provided data on the use of Penthrox for the following elective interventional procedures: (1) RCTs—nasoduodenal intubation for CT enteroclysis,18 colonoscopy,19 bone marrow aspiration and biopsy,20 prostate biopsy21 and outpatient hysteroscopy17 and (2) observational studies—prostate biopsy,22 24 27 colonoscopy,23 water vapour therapy for benign prostatic hyperplasia,25 gynaecological brachytherapy applicator removal28 and external fixator removal.26 9 of the 12 included studies were comparative, and the control groups included intravenous opioid analgesia (1), procedural sedation (2), local anaesthesia alone (2), local anaesthesia plus Penthrox (1) and placebo (3).
All included studies used 3 mL of methoxyflurane,17 18 20–28 but one used up to 6 mL.19 The timing of the medication administration varied with some studies commencing Penthrox inhalation between 1 and 5 min in advance of the procedure, and others starting inhalation during the procedure (tables 1 and 2). Common contraindications to the use of Penthrox included pre-existing renal or hepatic impairment (one study provided a specific definition: a creatinine >1.5 times upper limit normal or bilirubin ≥2.5 times upper limit normal20), use of concomitant nephrotoxic medications, possible personal or family history of allergy to medication or malignant hyperthermia or hypersensitivity to fluorinated agents, difficulty in following instructions, previous head injury and respiratory depression.
Study bias
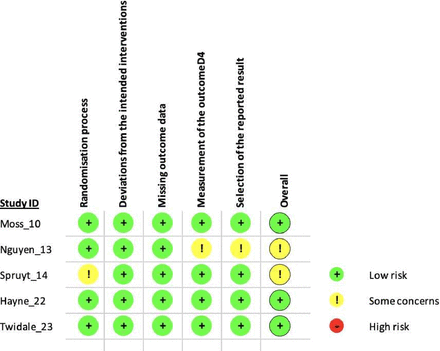
All of the bias assessments are presented in figure 2 (RCTs) and table 3 (observational studies). Three of the RCTs demonstrated a ‘low risk’ of concern,17 18 21 and two were considered to be of ‘some concern’ for bias mainly due to the lack of detail with regard to the selection of the reported result, measurement of the outcome and regarding the randomisation process.19 20 The seven non-randomised interventional studies were all graded at serious or critical risk of bias suggesting important limitations in the design and conduct of the studies mainly due to bias in the measurement of outcomes and confounding factors.22–28
Cochrane Collaboration Risk of Bias V.2.016 assessment of included studies.
Summary table of the risk of bias using Risk of Bias in Non-randomised Studies of Interventions (ROBINS I)15 for non-randomised studies of interventions
Pain
Randomised controlled trials
Table 4 outlines a summary of the primary outcome (pain) for all the RCTs evaluated in this review. Three RCTs used a 10 cm or 100 mm Visual Analogue Scale (VAS) to describe the pain score,17–19 while two RCTs used a Numerical Rating Scale (NRS) from 0 to 10.20 21 Four RCTs evaluated intraprocedural pain and measured the pain intensity at varying times after completion of the procedure.17–20 Three of these four RCTs compared Penthrox against placebo,17 18 20 while the fourth one evaluated Penthrox against intravenous sedation.19
Interventions, primary outcome assessment and procedural pain scores for randomised controlled trials
A statistically significant reduction in intraprocedural pain was demonstrated when patients undergoing bone marrow biopsy were randomised to Penthrox rather than placebo (3.3±2.0 (n=49) vs 5.0±2.4 (n=48); p<0.001).20 Similarly, for diagnostic and operative hysteroscopies, where patients were blinded to treatment allocation, a significant reduction in intraprocedural pain was identified when comparing Penthrox to placebo.17 The intraprocedural pain score for diagnostic hysteroscopy was 34.2±28.0 (n=45) vs 43.2±29.8 (n=44), p=0.05, and for operative hysteroscopy was 20.4±25.2 (n=35) vs 34.6±30.1 (n=32), p=0.03.17 A RCT looking at ‘patient comfort’ and using a 10 cm VAS where 0 represents ‘extremely uncomfortable’ and 10 represents ‘extremely comfortable’ during CT enteroclysis including nasoduodenal intubation did not identify a significant increase in overall comfort rating (4.8±3.1 (n=30) vs 4.0±2.7 (n=30); p=0.3) for Penthrox and placebo, respectively; however, there was a significant increase in patient comfort during the nasoduodenal intubation component of the CT enteroclysis procedure for patients who used Penthrox (5.0±2.7 (n=30) vs 2.7±2.5 (n=30); p=0.002).18 In the RCT where patients were randomised to either Penthrox or intravenous sedation for colonoscopy, no significant difference in intraprocedural pain was demonstrated19; unfortunately summary statistics was not provided despite contacting the first author for further information.
The fifth and final RCT evaluated postprocedural pain only, comparing Penthrox with placebo during prostate biopsies. The pain scores recorded were not statistically significant 15 min after the procedure (2.51±3.07 (n=209) vs 2.82±3.10 (n=211), p=0.2, or at 7–35 days postprocedure (2.56±3.49 (n=209) vs 2.79±3.38 (n=211); p=0.4) for Penthrox versus placebo, respectively.21
Non-randomised studies of interventions (NRSIs)
Table 5 outlines a summary of pain for the seven NRSIs evaluated in this review comprising four controlled and three uncontrolled cohorts. Four studies used a 10 cm VAS to describe the pain,23 25–27 one study used an NRS of 0–10,28 while the remaining two studies used a verbal rating scale of 0–10 to record the pain scores.22 24
Interventions, primary outcome assessment and procedural pain scores for non-randomised studies of interventions
Penthrox was evaluated against a control in three studies, which investigated intraprocedural pain, measured at varying times following completion of the procedure.
During brachytherapy applicator removal, there was a statistically significant reduction in pain with Penthrox when compared with intravenous paracetamol and/or morphine (median pain 0 (0–2) vs 7 (0–10); p 0.002).28 In contrast, a significant increase in pain was observed when Penthrox was used for pain relief during colonoscopy compared with intravenous sedation (3.6±0.2 (n=85) vs 0.9±0.1 (n=55); p<0.001), respectively.23 Similarly, when comparing Penthrox against local infiltration of anaesthetic for prostate biopsy, the median pain score was higher for Penthrox (4.0 vs 2.0; p=0.01 (n=11).27
Three studies compared Penthrox against a control investigating postprocedural pain. For prostate biopsy, the use of Penthrox alone compared with Penthrox with local anaesthetic infiltration was associated with significantly more pain (median pain score 3 (IQR 2–5) vs 2 (IQR 1–3); p 0.01 (n=72)) recorded immediately after cessation of the procedure.24 In contrast, another study showed a statistically significant reduction in the median pain score in favour of Penthrox when compared with intravenous paracetamol and/or morphine at 60 min following brachytherapy applicator removal (3 (0–10), n=12 vs 0 (0–2), n=7; p 0.04).28 Penthrox was also evaluated against intravenous sedation (fentanyl and midazolam) for colonoscopy, and the postprocedural pain level of patients was evaluated once they were alert and able to follow commands.23 However, details regarding the pain level were not available in the manuscript or following attempted contact with the first author.
Four studies reported pain score data following a single-arm cohort investigating the feasibility of using Penthrox during ambulatory procedures.22 25 26 28 These are detailed in table 5.
Anxiety
Randomised controlled trials
Anxiety was assessed in two RCTs using the ‘State Trait Anxiety Inventory’ systems (STAI-Y and STAI-Y-1),19 20 a validated self-report questionnaire measuring state and trait anxiety with scores ranging from 20 to 80 with a higher score correlating to higher anxiety levels. One further RCT collected data about patients’ fear of the biopsy using a bespoke ‘Patient’s experience of transrectal ultrasound (TRUS) prostate biopsy’ questionnaires.21
The STAI-Y anxiety scores were higher after rather than before colonoscopy. However, for the Penthrox group, this was reported as significantly higher than the intravenous sedation group, both precolonoscopy and postcolonoscopy (precolonoscopy: 46.2±0.5; n=125 vs 43.7±0.7; n-=126, p<0.05, and postcolonoscopy: 47.6±0.5; n=125 vs 45.4±0.7; n=126, p<0.05, respectively). However, this RCT reported a ‘nervousness score’, which they identified as a subscore derived from STAI-Y. Both Penthrox and intravenous sedation showed a significant reduction in the ‘nervousness score’ from baseline in patients undergoing colonoscopy (3.3±0.1; n=125, p<0.01 vs 4.5±0.2; n=126, p<0.01).19 No difference in the fear of prostate biopsy, assessed 15 min post procedure, was identified in patients randomised to Penthrox with periprostatic infiltration of local anaesthetic (PILA) compared with placebo with PILA (2.92±0.27 vs 3.06±0.27; p=0.6).21 A summary of the STAI Y-1 scores was not given in the study randomising patients undergoing a bone marrow biopsy to either Penthrox or placebo; however, the authors reported no statistical difference immediately post procedure and 30–45 min afterwards.20
Non-randomised studies of Interventions (NRSIs)
Anxiety was assessed in two studies: one used the STAI-Y score system postprocedure for patients undergoing colonoscopy and the other used their own bespoke questionnaire to assess patients’ anxiety postbrachytherapy applicator removal.23 28 Neither study reported their results, but both documented that there was no significant difference in anxiety level with the use of Penthrox compared with the use intravenous analgesia or procedural sedation.
Side effects
10 of the 12 included studies reported side effects associated with the use of Penthrox. The most commonly reported side effect during Penthrox inhalation across both the RCTs and observational studies was dizziness, reported in 4/12 (33%) studies with incidences ranging between 4% and 52%.20 21 Other side effects included a sickly sweet taste, nausea, cough, drowsiness, headache, vomiting and euphoria (online supplemental table 1). From a respiratory perspective, one study reported a patient who developed a mild wheeze, but with cessation of the medication and prophylactic administration of oxygen, their oxygen saturation remained >96% both before and after oxygen administration.18 Another study observed respiratory depression in five patients in the Penthrox group, but all these patients’ colonoscopies required additional intravenous sedation because they were not successful with Penthrox alone and respiratory depression occurred following this.19 This study also identified a small proportion of patients who presented with hypotension (4.3%) and tachyarrhythmia (2.6%) following Penthrox administration, but both these adverse events also presented in the sedation group19 (online supplemental table 1).
Supplemental material
Patient satisfaction or acceptability
10 studies reported on patient satisfaction and/or acceptability in terms of willingness to undergo a repeat procedure using Penthrox in the future. The satisfaction scores when presented as proportions ranged from 63% to >95% (seven studies). Eight studies reported the proportion of patients that would be willing to have the same procedure using Penthrox for pain control in the same circumstance in the future; seven showed that most patients would have the procedure again (range 60%–100%), but in one study of TRUS prostate biopsy with PILA and Penthrox, only the minority of patients would agree to a future procedure (46.8%)18–27 (online supplemental table 2).
Supplemental material
Clinician satisfaction and experience
One observational study assessing clinician satisfaction when using Penthrox during water vapour thermal therapy for benign prostatic enlargement reported a median clinician global satisfaction score of 100 (range 78.6–100) out of 10025 (online supplemental table 3). The three RCTs assessing clinician experience compared with alternative pain control measures showed either superiority21 or equivalence19 20 (online supplemental table 3).
Supplemental material
Discussion
Principal findings
The use of Penthrox (methoxyflurane) for invasive, elective interventional procedures in the outpatient setting to reduce pain has shown promise, although the relative benefits appear to vary according to the type of intervention and the comparator pain control measures. Two medium-sized RCTs showed a significant reduction in intraprocedural pain when Penthrox was compared with placebo for outpatient hysteroscopy as well as bone marrow aspiration and core biopsy, respectively.17 20 However, the other three RCTs investigating the use of Penthrox for nasoduodenal intubation, colonoscopy and TRUS biopsy were compared against placebo or intravenous sedation and showed no significant difference in pain or comfort level.18 19 21 It should be noted that Penthrox is rapidly eliminated from the plasma,8 and one of these negative RCTs21 assessed postprocedural pain 15 min after the procedure. Similarly, the NRSIs showed variable results, with Penthrox inferior to intravenous sedation for colonoscopy23 and local anaesthesia for prostate biopsies27 but significantly better compared with intravenous analgesia for brachytherapy applicator removal.28 Interestingly, for interventions such as colonoscopy and TRUS biopsies, which were looked at in both RCTs and NRSIs, there was no consistency in the results obtained with Penthrox. However, this was most likely due to differences in the study design and comparator. Where anxiety was assessed, most studies did not show any benefit of Penthrox. However, its use was associated with high levels of overall satisfaction, acceptability and a low side effect profile with no serious adverse events reported.
Strengths and limitations
This is the first systematic review to summarise the utilisation of the Penthrox inhaler outside of the emergency setting for the reduction of pain and anxiety during elective, invasive procedures. We formulated a clinically focused question by applying the population, intervention, comparator and outcome model, and we had a thorough search strategy by using multiple databases, not restricting to any languages, using broad search terms with no restrictions, scanning the reference lists of included studies to ensure no studies were missed and contacting authors, where required, for additional clarification or data. Selection bias was therefore minimised. Two independent reviewers, with guidance from a third reviewer if conflicts arose, identified the relevant studies, completed the data collection form and carried out the risk of bias assessment, hence reducing the risk of human error.
The main limitation of this review is the lack of large, high-quality RCTs for this question, and so we included other, non-randomised, interventional studies to maximise the data available. Since these studies were judged to be at an overall serious or critical risk of bias, caution should be exercised when interpreting the results because of inherent flaws in the design and conduct of these studies. With the methodological limitations in such study designs, it was not possible to perform a meta-analysis because of heterogeneity, including the type and timing of outcome assessment and types of interventions and controls that were assessed.
Comparison with existing literature
There are no other systematic reviews exploring the use of methoxyflurane for invasive elective procedures in the outpatient or ambulatory setting, reflecting the novelty of the use of Penthrox outside of acute, trauma settings. A recent narrative, literature review evaluated the use of Penthrox for procedural pain relief in cancer-related procedures29 with a slight overlap with our systematic review. They reported clinical equivalency or superiority for the use of Penthrox from the seven studies (three RCTs) they identified.
Implications for clinical practice and future research
With increasing pressure on the health services, especially in the post-COVID-19 pandemic era, it is imperative that outpatient settings are optimally used, to free up operating theatre space for more invasive procedures and help reduce the long waiting lists for surgery. Technological advances, especially in imaging and endoscopic instrumentation, have made outpatient interventions feasible, but pain and patient experience limit its wider use.30–32 Penthrox has shown promise as an option for pain relief which can enhance the tolerance and experience of planned invasive procedures. There are also data showing its utility for semielective procedures such as debridement and dressing of burns.33–35 Its analgesic and anxiolytic properties have a quick onset of action and a minimal impact on cardiorespiratory observations and recovery times.5 11 19 Thus, Penthrox has the potential to increase the efficiency of health services by facilitating outpatient procedures, providing a safe, easy to use, convenient and effective method of pain control while respecting patients’ freedom of choice as per the Comprehensive Model of Personalised Care.36
Currently, the evidence of efficacy for elective, outpatient procedures lacks generalisability and is too inconsistent to support the use of Penthrox in routine clinical practice. However, despite uncertainty about its effectiveness and cost-effectiveness, the use of Penthrox in elective settings is gaining traction.37 Robust research is urgently needed because there is a danger that Penthrox will be introduced indiscriminately into clinical practice without clearer evidence of benefits, harms and resource use. Assessment of this health technology requires large, multicentre RCTs with comparison against current standards of care and qualitative research to explore patient experience and acceptability.
In addition, if the use of low-dose methoxyflurane analgesia using a Penthrox inhaler is set to increase, further work to address concerns about potential hepatic and renal toxicity and occupational exposure may be warranted. It should be noted, however, that our review of studies using the recommended subanaesthetic doses of methoxyflurane in Penthrox did not report any such adverse events. One RCT did show the elevated fluoride level in some patients using Penthrox, but this was not in the toxic range.20 A literature review of the pathogenesis of renal damage in the context of methoxyflurane use concluded that the clinical use of Penthrox at an analgesic dose is not associated with renal adverse effects.9 A systematic literature review to identify potential health effects of Penthrox at analgesic dose on patients reported no increased risk of liver dysfunction or hepatotoxicity, though there were some isolated case reports of hepatitis which were believed to be an idiosyncratic response.10 38 To date, studies evaluating exposure of clinical staff to low-dose methoxyflurane analgesia via a Penthrox inhaler in emergency and elective settings have been reassuring where low levels of exposure, below minimum safe thresholds, have been reported.39 40
Conclusion
The application of methoxyflurane (Penthrox) as an effective, self-administered analgesic and anxiolytic in the emergency setting is well established. However, the technology is quickly gaining popularity for use in the outpatient and ambulatory setting for elective, invasive procedures. This popularity relates to its apparent efficacy, minimal side effect profile and ease of use, avoiding the need for cumbersome anaesthetic equipment and utilisation of scarce hospital resources. However, while there are some small, heterogenous RCTs available, this systematic review has highlighted a lack of good quality, generalisable evidence to draw meaningful conclusions regarding the use of Penthrox for specific indications. The need for safe, rapidly acting, effective and convenient pain control with quick recovery is clear as the number of outpatient procedures increases across all clinical specialties. More data in the form of adequately powered, robustly designed and standardised RCTs comparing the use of Penthrox (methoxyflurane), against the standard pain relief option for any elective procedure, are urgently required to support or refute its routine use in clinical practice.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
References
Footnotes
X @TJustinC
Contributors BZFS: review papers, risk-of-bias assessment and manuscript writing and editing. PMDS: review papers, risk-of-bias assessment and manuscript editing. PS: review papers, resolution of any disagreements and manuscript editing. TJC: conception of idea, project development, review papers and manuscript editing. BZFS is responsible for the overall content of the article as guarantor.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Author note The manuscript's guarantor affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned have been explained.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.