Article Text
Abstract
Background With the potential to identify a vast number of rare diseases soon after birth, genomic newborn screening (gNBS) could facilitate earlier interventions and improve health outcomes. Designing a gNBS programme will involve balancing stakeholders’ opinions and addressing concerns. The views of medical students—future clinicians who would deliver gNBS—have not yet been explored.
Methods We conducted a nationwide online survey of UK medical students via the REDCap platform. Perceptions of gNBS, including scope of testing and potential benefits and drawbacks, were explored using a mix of multiple-choice questions, Likert scales, visual analogue scales and free-text questions.
Results In total, 116 medical students across 16 universities participated. Overall, 45% supported gNBS, with a positively skewed mean support score of 3.24 (SD 1.26, range: 1.0–5.0), and 55% felt it relevant to their future practice. Almost all agreed that infant-onset and childhood-onset diseases and conditions with effective treatments should be included. Most felt that earlier interventions and personalised care would be the most important benefit of gNBS. Other perceived benefits included earlier diagnoses, diagnosing more patients and enabling research for new treatments. However, several perceived challenges were highlighted: risk of genomic discrimination, incidental or uncertain findings, data security and breaching children’s future autonomy. Students expressed conflicting opinions on the psychological impact on families, but most were concerned about a lack of support due to current resource limitations in health services. Students frequently reported having insufficient knowledge to form an opinion, which may reflect gaps in genomics education at medical school and the current lack of evidence base for gNBS.
Conclusion Although some support for gNBS was demonstrated, ethicolegal and social challenges were raised, emphasising a need for ongoing discussions about the implications of gNBS.
- GENETICS
- PUBLIC HEALTH
- PAEDIATRICS
- NEONATOLOGY
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. Raw data files can be made available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Our study design used an anonymous online survey, which enabled participants to freely share their honest opinions on genomic newborn screening.
Survey respondents were a representative sample of 116 participants from 16 different medical schools.
Medical students with more experience in the field of genomics or an interest in paediatrics may have been more likely to participate in this study.
As this was an anonymous survey study, we were unable to ask participants follow-up questions to further explore nuances to their views or individual reasons that inform their opinions.
Introduction
The aim of newborn screening is to identify infants with an inherited or congenital condition before symptoms manifest and enable earlier management, improving health outcomes and quality of life for patients and families. Newborn screening typically includes a blood spot test that screens a small number of rare conditions in which early identification and treatment have proven to be beneficial.1 The conditions screened vary between countries—several US states screen for over 50 conditions,2 numerous countries in the European Union screen for around 30,3 whereas the UK screens for only nine.1 Over the last decade, healthcare professionals (HCPs) and members of the rare disease community have been calling for newborn screening programmes to expand.4
As the cost of DNA sequencing has fallen and the diagnostic power of whole genome sequencing (WGS) in rare disease has been demonstrated in recent years,5 interest in employing genomic newborn screening (gNBS) has grown. With the potential to identify a vast number of rare diseases soon after birth, gNBS could facilitate earlier intervention and improve health outcomes in a larger number of cases than current newborn screening programmes and may ultimately alleviate the significant population health and economic burdens rare diseases exert collectively.6 Indeed, the first research studies employing gNBS have already been performed in several high-income countries—the BabySeq project in the US and Baby Beyond Hearing project in Australia have both used exome sequencing.7 8 Other large-scale gNBS projects are being launched worldwide,9 including the UK-based Generation Study, which aims to perform WGS for 100 000 newborns.10
In addition to demonstrating clinical benefits, an effective screening programme hinges on its acceptability to a range of stakeholders, including parents, the public and HCPs11; designing an effective gNBS programme will involve balancing stakeholders’ views and addressing their concerns.12 A public dialogue commissioned by the UK National Screening Committee, Genomics England and the UK Research and Innovation’s Sciencewise programme recently found high levels of support among the UK public on the use of WGS in routine newborn screening.13 Similarly, the Australian public is generally supportive of introducing genomic sequencing into newborn screening.14 Parents and expectant parents in North America and Australia have also expressed support for gNBS, particularly regarding receiving information on actionable childhood conditions,15–20 whereas HCPs tend to be more reserved in their support for gNBS compared with parents. Parents have raised concerns regarding the accuracy of results, the need for accessible information for them to make informed decisions17 18 and the importance of extensive consent processes.20 HCPs have expressed ethicolegal concerns surrounding genetic discrimination and preserving children’s autonomy,15 as well as practical concerns like data privacy and storage costs, the burden of variants of uncertain significance and diseases with variable penetrance.12 In addition, there is a lack of consensus among both parents and HCPs on which conditions should be included in gNBS.12 These concerns and associated differences in support for gNBS emphasise the need to generate high-quality evidence in large-scale research studies, as well as for continued discussions with diverse stakeholders, to inform public health decisions regarding implementation of a gNBS programme.9
One stakeholder group whose opinions on gNBS have not yet been explored is medical students, who will constitute the future medical workforce that may deliver gNBS as part of standard care. Here, we aim to explore the opinions of UK medical students on the proposed use of WGS in newborn screening.
Methods
We conducted a cross-sectional study, by disseminating an online survey to UK medical students in the final 2 years of the study.
Survey design
The survey design has previously been reported.21 In brief, the survey was developed by a multidisciplinary research group (comprising medical students, a social scientist, a clinical geneticist and a neonatologist), reviewed by the Clinical Director of Health Education England’s Genomics Education Programme and the Clinical Lead for Genetic Counselling at Genomics England and piloted through ten cognitive ‘Think Aloud’ interviews.22
The final survey was organised into three sections: demographic information, genomics educational experience and knowledge and views on newborn screening programmes (online supplemental material 1). Findings from the genomics educational experience and knowledge section are published elsewhere.21 The newborn screening programme section consisted of 43 questions. Participants were first asked whether they were aware of the proposed study employing WGS in newborn screening (Generation Study). Using five-point Likert scales, participants were asked to rate: their level of agreement with ten statements about newborn screening (ranging from ‘Completely disagree’1 to ‘Completely agree’4 with an option of ‘Not enough knowledge’); whether six categories of diseases should be included in gNBS (ranging from ‘Completely disagree’,1 ‘Neutral’3 to ‘Completely agree’5); and the importance of seven potential benefits and ten potential drawbacks of gNBS (ranging from ‘Not important’1 to ‘Very important’5). Participants were also asked to identify the most important potential benefit and drawback from the list of options and had the opportunity to provide free-text responses to describe any additional potential benefits or drawbacks. In a multiple-choice question where participants could tick all options that applied and leave a free-text response, they were asked which groups of people should have access to the raw data generated by gNBS. Using visual analogue scales, participants were asked whether overall they supported the introduction of WGS into newborn screening and how relevant they thought a potential gNBS programme would be to their future medical practice. Participants were given the opportunity to leave a free-text response explaining their answer to these questions or to leave any final thoughts on the subject.
Supplemental material
Survey dissemination
The survey was hosted on the web-based platform, REDCap.23 24 Invitations to complete the survey via an anonymous link were emailed to all UK medical schools on 14 November 2022 and the Medical Schools Council disseminated the survey on 6 January 2023. The survey was also shared via the Great Ormond Street Hospital Postgraduate Medical Education Department’s social media platform and the mailing lists of several university paediatrics societies. The survey was closed on 24 February 2023.
Data analysis
Data analysis was performed in RStudio.25 The responses from the five-point rating scales were combined into ‘Disagree’ or ‘Not important’1 2 and ‘Important’ or ‘Agree’ (3–4 or 4–5). All responses to free-text questions were aggregated and analysed collectively. Common themes within the text were identified and coded in an iterative process using summative thematic analysis.26 Exemplar quotes for the identified themes are reported.
Patient and public involvement
None.
Results
Participant characteristics
A total of 116 complete responses, 75 of which included responses to free-text questions, were received from medical students at 16 different UK medical schools, the demographic details of which are given in table 1. Only 16% (n=18) of students were aware of the proposed newborn WGS study (Generation Study) before participating in this study.
Participant characteristics
Opinions on newborn screening
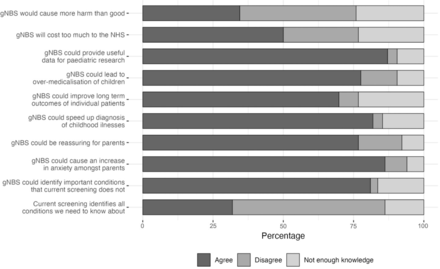
Just over half (54%) did not think the current newborn blood spot screening programme was sufficient to detect all early-onset childhood conditions that medical professionals need to know about, and even more (81%) agreed that the introduction of gNBS could identify important conditions that the current screening programme does not (figure 1). However, while 77% of respondents agreed that gNBS could be reassuring for parents, 86% thought that it could increase unnecessary anxiety among new parents regarding their child’s health. Approximately one-third (34%) of participants agreed with the statement that gNBS ‘would cause more harm than good’.
Distribution of medical students’ level of agreement with statements about newborn screening. gNBS, genomic newborn screening; NHS, National Health Service.
Scope of gNBS and data access
The majority of participants agreed that diseases that typically manifest during the first year of life (94%) or in childhood (86%) should be included in gNBS; diseases with later onset garnered less support (figure 2a). The availability of effective treatments for a disease also influenced support for inclusion—almost all participants (95%) agreed that diseases for which there is an effective treatment available should be included, whereas only 42% supported the inclusion of diseases for which treatment is limited and supportive care is the primary option.
Respondents’ agreement with the inclusion of different categories of disease in a newborn WGS screening programme (a) and opinions on which groups should have access to data generated by a newborn WGS screening programme (b). WGS, whole genome sequencing.
Most respondents thought that clinicians directly involved in the patient’s care (97%) should have access to data generated from gNBS (figure 2b). Three-quarters of participants (75%) thought that National Health Service (NHS)-affiliated research groups should have data access, compared with only 18% who thought that the data should be made available to external research groups, such as those affiliated with pharmaceutical companies (figure 2b).
Potential benefits and drawbacks of gNBS
Nearly all respondents agreed that being able to diagnose conditions earlier (95%) and to provide earlier interventions and personalised care (95%) were important potential benefits of gNBS (figure 3a), with 70% of participants identifying the latter as the most important potential benefit (figure 3b). Being able to diagnose more patients (84%) and facilitating research into new treatments (89%) were also seen as important potential benefits by most (figure 3a). In contrast, only 20% regarded the ability to create a lifetime genomic record for patients important (figure 3a).
Importance ratings for potential benefits (a) and drawbacks (c) of a gNBS programme and medical students’ perception of the single most important benefit (b) and drawback (d) of a gNBS programme. gNBS, genomic newborn screening; NHS, National Health Service.
The free-text responses relating to the additional potential benefits of gNBS fell under four sub-themes (table 2). Several participants noted opportunities for basic research in genetic disease, with one participant commenting that performing research with gNBS data may provide a ‘greater understanding of pathogenesis of diseases, not just about treatment’ (Participant 102). Benefits for the family unit were also identified—parental acceptance of diagnosis and providing important information for allowing family planning decisions.
Themes identified in qualitative responses to questions regarding gNBS. Participants were asked to describe potential benefits and drawbacks, in addition to those that had been explicitly asked about elsewhere in the survey which are not included here.
The vast majority of respondents felt the lack of resources in the NHS to support families (94%) and the potential for future discrimination based on genomic information (93%) were important potential drawbacks of gNBS (figure 3c). Almost one-third (30%) of participants identified the latter as the most important potential drawback (figure 3d), and this concern was echoed in the free-text responses where many respondents described concerns of potential discrimination. A large proportion of respondents also found the risk of incidental or uncertain findings (87%) and the risk of false reassurance (70%) ‘important’ potential drawbacks.
Many respondents expressed apprehension surrounding the privacy and security of genomic data—‘information from WGS has the potential to end up in unintended places. That risk is worth avoiding in the first place’ (Participant 20) and ‘The risk of a security breach is too high’ (Participant 45)—and 85% rated it an ‘important’ potential drawback of gNBS (figure 3c).
Despite nearly half (47%) rating the potential for gNBS to interfere with the early bonding process as ‘not important’, over three-quarters (78%) felt the emotional impact on parents/carers in receiving an early genetic diagnosis in a child who otherwise appears healthy was an important concern (figure 3c). Themes surrounding the negative psychological impact on both the parents and the child frequently emerged in free-text responses, with participants raising concerns that the impact on the parent–infant relationship may be so significant as to result in parental neglect (table 2).
Parents consenting to WGS on behalf of their newborn was seen as an important drawback by 69% of participants. Qualitative responses falling under the theme of removal of patient autonomy (table 2) argued that gNBS constitutes ‘a breach of the child’s future autonomy… there is potential to do psychological and autonomous harm’ (Participant 76).
Overall gNBS support and relevance to future practice
Overall, 45% supported the introduction of gNBS to the current newborn screening programme, and the distribution of support scores was skewed to the right, with a mean score of 3.24 (SD 1.26, range: 1.0–5.0) (online supplemental figure). Many participants reported that they were unable to reach a conclusion about supporting gNBS because of a lack of knowledge, with one participant writing ‘I do not have enough information about this to decide whether or not I would support’ (Participant 10). Just over half (55%) felt that gNBS will be relevant to their future medical practice, with a mean overall rating of 3.7 (SD 2.87, range: 1.0–5.0). The median rating was 4.0, which was selected by 20% of participants, and 35% selected the maximum relevance rating of 5.0 (online supplemental figure).
Supplemental material
Discussion
This study provides the first insight into UK medical students’ attitudes towards gNBS. Medical students were positive about the potential benefits of gNBS but also raised concerns. Overall, just under half explicitly supported its introduction to newborn screening, and just over half felt it will be relevant to their future practice. This echoes the general cautious stance of HCPs15 27–29—a recent study of Australian HCPs revealed that although most had reservations about the use of gNBS currently, the majority foresee its use by 2026,20 and US doctors also expect gNBS to be more useful 5 years from now.15 Some of the ambivalence towards gNBS expressed by medical students herein may be explained by a lack of knowledge—many students indicated that they did not have enough knowledge to decide whether they agreed with various statements about gNBS and this issue was raised by several students in their free-text responses qualifying their level of gNBS support (figure 1). This may be a reflection of the low levels of confidence in genomic knowledge that was also revealed among these participants, where we concluded that robust genomic education through undergraduate medical curricula is necessary to equip the future workforce to deliver genomic medicine.21 The medical students’ apprehension may also be borne out of the lack of evidence currently available about the clinical benefits of gNBS, as large-scale research evaluating gNBS is in its infancy. This emphasises the importance of the large prospective gNBS studies that are launching across Europe, North America and Australia to generate robust evidence that can inform decisions regarding implementation of gNBS programmes in the near future.9
The most important benefits purported by medical students were its potential to facilitate earlier diagnoses and interventions in more patients, with 82% agreeing that gNBS would speed up diagnoses. Similar views were shared by numerous participants in a recent qualitative study of Australian HCPs with clinical or policy experience in newborn screening or genomic sequencing.29 Furthermore, most students felt that current newborn screening is insufficient at detecting clinically important, early-onset childhood conditions and even more agreed that gNBS could deliver where current screening does not. Although numbers with a diagnosis were small, the results from the US BabySeq project, a randomised study that tested for around 1000 childhood-onset conditions and five adult-onset cancer predisposition syndromes, support this view.30 Of the 15 infants that were identified as at-risk of childhood-onset disease and the three at-risk of adult-onset disease, only two infants were diagnosed through standard newborn screening and not exome sequencing, whereas 15 infants were diagnosed through exome sequencing and not standard newborn screening, thus demonstrating the potential value of gNBS.31
Despite expressing some support for gNBS, medical students revealed several concerns consistent with those reported among HCPs and parents in the literature: privacy and data security, uncertainty of results and incidental findings and discrimination on the basis of genomic information.15 16 18 20 29 In the free-text responses, medical students frequently elaborated their concerns around security of genomic data, and Cao et al reported HCPs frequently sharing anecdotes of parental concerns about storage of dried bloodspot cards that they anticipate will increase with storage of genomic data.29 Indeed, 13% of parents who declined recruitment into the BabySeq project did so due to concerns of privacy and discrimination.32 This indicates a level of distrust in institutions responsible for protecting genomic data from unauthorised access that is consistent among medical students, HCPs and parents across different countries with different healthcare systems that should be addressed. Furthermore, with almost one-third of medical students identifying the risk of genomic discrimination as the most important potential drawback, robust international legislation to safeguard against the misuse of genetic information is required, as recently highlighted by HCPs,20 for the future implementation of any screening programme employing genomic sequencing modalities.
Medical students expressed conflicting views on the psychological impact of an early diagnosis on the family, and this theme frequently emerged in free-text responses. Over three-quarters of medical students felt that gNBS could be reassuring, a view shared by parents in another study.17 However, over three-quarters of medical students were also concerned about the emotional impact on parents receiving an early diagnosis in a child who otherwise appears healthy and felt that gNBS could increase unnecessary anxiety. Many shared concerns pertaining to the impact on the parent–infant relationship and risk of parental neglect or abandonment. Potential for psychological distress is a frequently raised concern around gNBS among HCPs and parents in the literature15—in a recent survey, Slovenian peripartum mothers shared that their attitude towards their family and relationship with their partner would suffer if they knew their child was a carrier of an incurable genetic condition.33 However, evidence from two of the few studies that have employed gNBS to date suggests these concerns may not materialise among those who are willing to undergo gNBS—the BabySeq Project reported no persistent negative psychosocial effects among families who received gNBS,34 and the Baby Beyond Hearing Project found no evidence that offering different levels of genomic information to parents has a negative psychological impact.8 Further evaluation in larger prospective studies, involving a diverse population of parents from a range of backgrounds and who receive a range of different results, is warranted to better elucidate the psychosocial impact of gNBS. Of note, the UK public dialogue found support for gNBS, provided that mental health services are available to provide emotional and psychological support for those who receive a diagnosis.35 With almost all medical students in our study highlighting the current lack of resources in the NHS to support families as an important potential drawback of gNBS, this is a very important consideration that needs to be addressed with multi-disciplinary input in the design of possible future services.
There is currently no consensus in the literature on the scope of conditions to be tested for using gNBS. The vast majority of medical students supported screening for childhood-onset conditions, as do most HCPs and parents in recent studies.16 17 19 20 29 Consistent with the views of HCPs,20 few medical students supported screening for adult-onset conditions, unlike those of most parents who have still supported screening for such conditions.16 Much like HCPs in recent studies,15 20 many medical students raised concerns about gNBS breaching the child’s future autonomy to decide whether this information is wanted as an adult and most saw parents consenting to WGS on behalf of their newborn as an important potential drawback of gNBS. Indeed, to mitigate some of these risks, it has been proposed that gNBS warrants a more extensive consent process compared with current newborn screening programmes,20 27 and parents have raised concerns about receiving accessible information to make an informed decision.17 However, this consideration will need to be balanced with the availability and associated financial costs of providing the trained professionals required to perform this at scale,16 20 36 which may limit the screening programme’s efficiency, and with the risk of reducing participation rates,32 which may compromise the screening programme’s effectiveness. Digital decision aids may alleviate some of this additional workload without compromising quality or worsening existing disparities,37 and a tablet-based decision aid was implemented in the recent North Carolina Newborn Exome Sequencing for Universal Screening randomised control trial and will be evaluated soon.38 39
Furthermore, contrary to most parents in several recent studies who also supported screening for conditions that cannot be prevented, treated or cured,16 17 19 fewer than half of the medical students in this study supported screening for conditions with only supportive care options. The interplay of the age of onset with actionability appears to determine HCPs’ opinions—68% supported screening for childhood-onset non-actionable conditions compared with 31% for adult-onset non-actionable conditions in a recent study.20 Screening for conditions with limited actionability presents a potential conflict with one of Wilson and Junger’s classical principles for population screening—an accepted treatment should be available for a condition screened.11 ‘Treatment’ in the context of monogenic conditions can include a wide range of interventions, beyond direct management of the condition in the index patient. This was exemplified in Green et al’s analysis of the actionability of pathogenic and likely pathogenic variants identified in the BabySeq project—identification of these variants directly prompted a specialty referral, surveillance or treatment in two-thirds of infants and their first-degree family members.40 Indeed, frameworks for evaluating genomic testing in population screening, based on modified Wilson and Junger criteria, have been developed to guide the development of such programmes.36 41–43 Continual assessment in the context of the availability and accessibility of healthcare in the country implementing gNBS will be required, especially as targeted treatments for rare diseases advance.
Our results should be regarded within the limitations of this study. With two-fifths of respondents having additional genomics experience beyond the core undergraduate medical curriculum, the demographics of participants may not be representative of the UK medical student population and those with more experience in the field may have a greater awareness of some of the complex issues surrounding gNBS. The design of this study is also a limitation—even with free-text response questions, it was not possible to comprehensively capture all nuances to medical students’ views or explore reasons that inform such views in an online survey. Future research could consider qualitative interviews to gather a more in-depth understanding of medical students’ views.
As future clinicians, medical students’ views on gNBS are important to inform the future design of such programmes. This is the first study to explore medical students’ opinions on gNBS. Students expressed positive views on gNBS, emphasising its potential to facilitate faster diagnoses and treatments in more patients. However, important ethical, legal and social challenges were raised, namely, data security, uncertainty of results, genomic discrimination and the psychological impact on families, as echoed by HCPs in the literature. Medical students also highlighted a lack of knowledge to form opinions on various aspects of gNBS, emphasising the need for sufficient education on genomics during medical school as well as good quality evidence from large prospective gNBS studies that are currently underway.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. Raw data files can be made available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by UCL Research Ethics Committee [Project ID: 23129.001] and the Clinical Research Adoptions Committee, UCL Great Ormond Street Institute of Child Health. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We are very grateful to all of the medical students who took part in the survey. Thank you to Marissa Willock, Postgraduate Medical Education Department at Great Ormond Street Hospital, for supporting medical student research and facilitating medical students to join research placements at Great Ormond Street Hospital. MP and MH are partially funded by the NIHR Biomedical Research Centre at Great Ormond Street Hospital. All research at Great Ormond Street Hospital NHS Foundation Trust and UCL Great Ormond Street Institute of Child Health is made possible by the NIHR Great Ormond Street Hospital Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the UK Department of Health.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
CSdC and MH are joint senior authors.
Contributors LS is the guarantor of the study, accepts full responsibility for the finished work and/or the conduct of the study, had access to the data and controlled the decision to publish. LS devised the concept for the study. All authors contributed to the conception and design of the study. AS and MP performed statistical analysis. AS, CS and MH performed thematic analysis. LS wrote the manuscript. AS contributed to the manuscript. AP, MP, ST, CS and MH reviewed and revised the manuscript critically. All authors collaborated on manuscript revision and read and approved the submitted version.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests AP is an employee of Genomics England, a company wholly owned by the United Kingdom Department of Health and Social Care and funded to deliver the Generation Study.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.