Article Text
Abstract
Objectives To investigate the association between multimorbidity during pregnancy and neurodevelopmental delay in offspring using data from a Japanese nationwide birth cohort study.
Design This study was a prospective birth cohort study.
Setting This study population included 104 059 fetal records who participated in The Japan Environment and Children’s Study from 2011 to 2014.
Participants Pregnant women whose children had undergone developmental testing were included in this analysis.
Primary and secondary outcome measures Neurodevelopment of offspring was assessed using the Japanese version of the Ages and Stages Questionnaire, third edition, comprising five developmental domains. The number of comorbidities among the pregnant women was categorised as zero, single disease or multimorbidity (two or more diseases). Maternal chronic conditions included in multimorbidity were defined as conditions with high prevalence among women of reproductive age. A multivariate logistic regression analysis was conducted to examine the association between multimorbidity in pregnant women and offspring development.
Results Pregnant women with multimorbidity, single disease and no disease accounted for 3.6%, 30.6% and 65.8%, respectively. The ORs for neurodevelopmental impairment during the follow-up period were similar for infants of mothers with no disease comorbidity and those with a single disease comorbidity. However, the ORs for neurodevelopmental impairment were significantly higher for children born to mothers with multimorbidity compared with those born to healthy mothers.
Conclusion An association was observed between the number of comorbidities in pregnant women and developmental delay in offspring. Multimorbidity in pregnant women may be associated with neurodevelopmental delay in their offspring. Further research is required in this regard in many other regions of the world.
- PUBLIC HEALTH
- Maternal medicine
- Paediatric neurology
- Multimorbidity
- Pregnant Women
Data availability statement
Data may be obtained from a third party and are not publicly available. Data are unsuitable for public deposition due to ethical restrictions and legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amendment on 9 September 2015) to publicly deposit the data containing personal information. Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restrict the open sharing of the epidemiologic data. All inquiries about access to data should be sent to: jecs-en@nies.go.jp. The person responsible for handling enquiries sent to this e-mail address is Dr Shoji F. Nakayama, JECS Programme Office, National Institute for Environmental Studies.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The study size was adequate for effective investigation.
Neurodevelopmental progress was assessed in detail using the results of eight points (6 months, 1 year, 1.5 years, 2 years, 2.5 years, 3 years, 3.5 years and 4 years).
Chronic diseases that were diagnosed but not treated were ruled out.
Infants were unable to communicate well, which renders accurate assessment of their neurodevelopment difficult.
Introduction
Multimorbidity is defined as the coexistence of two or more chronic diseases, whether physical or mental, in the same individual.1 Multimorbidity is considered one of the principal challenges in older people as the incidence of chronic diseases, such as hypertension, dyslipidaemia, diabetes, cardiac disease and malignant tumours, increases with age. Therefore, many studies have focused on older patients with multimorbidities.2 3 However, diseases such as asthma, arthritis, mental disorders and HIV can also occur in young people. There are few studies on multimorbidity in young people,4 including pregnant women.5 6 Maternal physical morbidities, such as hypertension, kidney disease and systemic lupus erythematosus, are potential risk factors for preterm birth (PTB) and low-birth weight infants (LBW).7 Moreover, maternal mental and social morbidities have also been associated with PTB and LBW.7 Previous studies also reported the relationship between maternal environments such as maternal asthma, maternal intake of fats, maternal and cord blood Manganese levels and child development.8–10
Infancy is considered to be the period in which language, cognition, motor skills and socioemotional domains form the basis for subsequent social participation.11 It is essential to receive appropriate support, early detection and intervention during this period.12 Although maternal nutritional status, certain diseases and blood substances can affect the neurodevelopment of offspring,8–11 the impacts of multimorbidity in pregnant women on the neurodevelopment of offspring have not been extensively studied.5 6 A major difference between previous reports and this study was the investigation of the association between multiple diseases of pregnant women and child neurodevelopment; previous reports have mainly focused on the relationship between a single disease or single substance in pregnant women and child neurodevelopment.
The present study aimed to investigate the association between multimorbidity during pregnancy and neurodevelopmental delay in offspring (every 6 months from birth to age 4 years) using data from an ongoing nationwide birth cohort, namely the Japan Environment and Children’s Study (JECS)13; the neurodevelopment of the participants was evaluated using the Japanese version of the Ages and Stages Questionnaires, Third Edition: Infant Developmental Examination (ASQ-3).14
Methods
Study population
The JECS is a nationwide and government-funded birth cohort study that started recruiting expecting mothers in January 201113; the primary objective was to investigate environmental factors such as exposure to chemicals and airborne pollutants that can affect children’s health and development during the fetal stage and early childhood, in order to help policymakers to formulate measures to safeguard the environment for future generations.15 The study population included 104 059 fetal records who participated in JECS from 2011 to 2014. A flowchart of the study participants is presented in figure 1. The exclusion criteria included miscarriage, stillbirth or unknown birth outcomes (n=2123). Second, participants with multiple births, pregnancies with chromosomal abnormalities, participated for the second time and more, and missing information about drug history, domestic violence, maternal infection or maternal body mass index (BMI) were excluded (n=13 377). Moreover, pregnant women whose children were not tested using the ASQ-3 once from 6 months to 4 years old (n=4046) were excluded. Finally, a total of 82 877 pregnant women were included in the analysis.
Fetal records selection flowchart. ASQ-3, Ages and Stages Questionnaires, Third Edition: Infant Developmental Examination.
Patient and public involvement statement
This study did not involve patients or public.
Assessment of pregnant multimorbidity
In this study, multimorbidity was defined as the coexistence of two or more physical, mental or social conditions in an individual according to previous reports.7 Maternal chronic conditions included in multimorbidity were defined as conditions with high prevalence among women of reproductive age.7 To identify pregnant women with the disease more rigorously, the diseases of pregnant women were defined as those that were medically treated at the time of pregnancy. Information was collected through self-reports, medical record transcripts and medication interviews. The targeted diseases included allergic diseases, such as asthma, anaemia, diabetes mellitus, dyslipidaemia, epilepsy, gastric or duodenal ulcers, heart disease, hepatitis, HIV infection, hypertension, inflammatory bowel disease, kidney disease, malignancy, migraine, neurologic disease, other sexually transmitted diseases (Chlamydia trachomatis and syphilis), mental disorders, rheumatic or collagen diseases and thyroid disease. Having an episode of domestic violence, substance abuse, being obese (BMI≥25), and being thin (BMI<18.5) were each defined as one disease. We used maternal prepregnancy body weight data for analysis. Pregnant women with two or more of these diseases during pregnancy were defined as having multimorbidities.
Assessment of neurodevelopment of offspring
Score results from the Japanese version of the ASQ-3 (Infant Development Test) at 6 months, 1 year, 1.5 years, 2 years, 2.5 years and, 3 years, 3.5 years and 4 years were used to evaluate neurodevelopmental measures.15 These scores were obtained by mailed questionnaire survey filled by caregivers. Neurodevelopmental assessments were performed in the domains of communication, gross motor, fine motor, problem-solving and personal–social. Offspring with scores below the cut-off were defined as having neurodevelopmental delays. The cut-off values were those reported in the Japanese validation version.14
Covariates
The covariates were maternal age at birth, parity, alcohol consumption status, smoking status, educational attainment, household income and sex of the child; they were selected based on previous studies.7 10
Statistical analysis
This study used the datasets jecs-ta-20190930 and jecs-qa-20210401 from JECS. STATA (MP17) and R (V.4.2.2) were used for statistical analysis. Multivariate logistic regression analysis was performed to determine the adjusted ORs. The objective variable was the neurodevelopment of the offspring, and the explanatory variable was the multimorbidity in pregnant women. The covariates were maternal age at birth, alcohol consumption status, smoking status, educational attainment, household income, sex of the child and number of births. Multiple imputation methods were performed using R to impute the missing values. Other analyses were performed using the STATA software.
Results
The characteristics of the pregnant women analysed in this study are presented in table 1. Pregnant women with multimorbidity, single disease and no disease accounted for 3.6% (n=3001), 30.6% (n=25 341) and 65.8% (n=54 535), respectively. Household income of 2–7.99 million/year was accounted for 84.7%; n=70 184. In total, 51.4% (n=42 563) and 48.6% (n=40 314) of the offspring were men and women, respectively. After pregnancy, 4.1% (n=3, 408) and 2.7% (n=2, 253) of pregnant women had smoking and drinking habits, respectively.
Characteristics of pregnant women and their offspring (n=82 877)
The prevalence of 23 maternal diseases is described in online supplemental table 1. Maternal underweight (BMI<18.5) (15.6%) was the most frequently observed chronic condition, followed by maternal obesity (BMI≥25) (10.7%). The most frequent diseases on medication were allergic diseases (3.1%), other sexually transmitted diseases (1.3%), anaemia (0.7%), mental disorders (0.7%) and thyroid disease (0.7%).
Supplemental material
The prevalence of neurodevelopmental delay in offspring is presented in table 2. The prevalence of communication delays at 6 months and 1 year was significantly lower than that of the others.
Prevalence of neurodevelopment delay of offspring
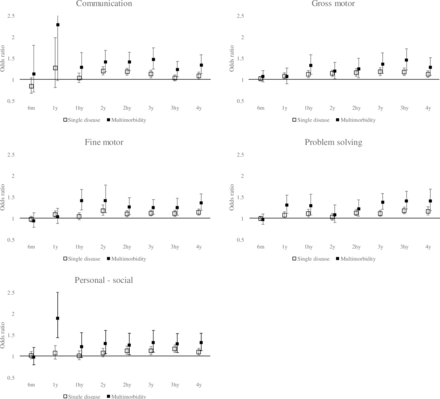
The number of the offspring tested as well as the mean ASQ-3 scores at each time point in the offspring those were analysed and those who were excluded are shown in online supplemental table 2. In the included group, the number of the offspring tested at 6 months and 4 years were 74 195 and 65 705, respectively. In the excluded group, the number of the offspring tested at 6 months and 4 years were 9642 and 9019, respectively. At each time point, the offspring were defined as tested if they answered at least one domain of the ASQ-3. The examination rates in offspring who were excluded were lower overall. The number of the offspring tested tended to decrease with age in both groups. The difference in the mean scores of the offspring excluded from the mean scores of those included ranged from −2.44 to 0.11. The mean scores in the offspring who were excluded were lower from 6 months to 4 years in most time points. The ASQ-3 scores and the number of the offspring by categories of the number of tests at each time point are shown in online supplemental table 3. The offspring were categorised into three groups: until 4 years, tested in all time points, 1 to 3 times, and 4 to 7 times. The number of the offspring tested at all time points, 4 to 7 times and 1 to 3 times were 46 766, 26 578 and 9530, respectively. The number of the offspring tended to decrease with age in groups tested less frequently. There was a particularly large decrease in the group tested 1–3 times. The difference in ASQ-3 scores of the groups tested less frequently from those of the group tested in all time points ranged from −1.62 to 3.37. Comparing the group tested in all time points, the groups tested less frequently tended to have higher scores until 2 years and lower scores after 2.5 years. The results of the multivariate logistic regression analysis conducted on the number of comorbidities in pregnant women and the neurodevelopment of offspring are shown in table 3 and figure 2. Except at 6 months, the ORs were more than one for any of the following items: communication, gross motor, fine motor, problem-solving and personal and social. The ORs at 6 months were lower than those at other ages for all items, both single disease comorbidity and multimorbidity. ORs tended to be higher with increasing age of the offspring, and the ORs for all items were higher at 4 years than at 6 months for both single-disease coexistence and multimorbidity. The ORs for single disease comorbidities ranged from 0.85 (95% CI 0.69 to 1.05) to 1.28 (95% Cl 0.82 to 1.99). The OR range for multimorbidity was 0.95 (95% CI 0.80 to 1.14) to 2.29 (95% CI 0.98 to 5.36), and that at 4 years of age was 1.30 (95% CI 1.11 to 1.52) to 1.42 (95% CI 1.19 to 1.69) for all domains.
Supplemental material
Supplemental material
Adjusted OR for developmental delay of offspring for multimorbidity during pregnancy by logistic regression
Adjusted OR for developmental delay of offspring for multimorbidity during pregnancy by logistic regression. Models were adjusted for maternal age at birth, parity, history of alcohol consumption, history of smoking, maternal educational attainment, household income and sex of child. Error bars indicate 95% CIs. The 95% CI for communication at 1 year with multimorbidity was 0.98 to 5.3.
Discussion
This investigation showed significant associations between multimorbidities in pregnant women and delayed neurodevelopment in the offspring. The ORs were higher for most of the neurodevelopmental items in pregnant women with multimorbidities than in those with a single disease. This study is the first to highlight the significance of the association between multimorbidity in pregnant women and the neurodevelopment in the offspring, despite the existence of reports on the association between specific diseases, such as asthma, chronic inflammatory arthritis, depression, thyroid conditions, diabetes and epilepsy, in pregnant women and the neurodevelopment of their children.8 16–18 As the number of comorbidities in pregnant women increases, the factors contributing to neurodevelopmental delay in the offspring may increase. In the future, health education and treatment in terms of the number of comorbidities during pregnancy should be considered.
The ORs for neurodevelopmental delay increased with the increase in the offspring’s age. This may have been caused by the increasing accuracy of the assessment as the offspring aged. An accurate assessment of neurodevelopment cannot be made until the child has grown to a certain age.19 Parents’ assessments of their children’s neurodevelopment may not be established until a certain period of parenting time. Neurodevelopmental delays may have been caused by social factors.20 It has been reported that depressed mothers tend to form family environments that are socially and economically disadvantageous to their children.21 Pregnant women with multimorbidities and certain mental diseases may have tended to form socioeconomically undesirable family environments.22 Furthermore, a great deal of the brain’s ultimate structure and capacity is shaped up to 3 years of age.11 The maternal immune activation may be caused by comorbidities during pregnancy, and components of the maternal immune system such as microglia and cytokines produced by microglia may trigger inappropriate fetal immune responses and may lead to neurodevelopment delay in the future.23 Neurodevelopmental delays in children may have gradually appeared as a result of multiple factors such as the postnatal brain development process, the undesirable family environment and the caregiver’s assessments of their children. Future research should take into account the prospect that factors such as children’s birth weight and/or gestational age at birth, nutritional status, Apgar score and maternal psychological status can be intermediate variables in the association between multimorbidity and neurodevelopmental delay.
This study has several limitations. First, pregnant women with diagnoses but no medication were not included in the disease sample in this study, with the exception of domestic violence, obese and skinny women. The criterion for the disease was defined as the presence of medication; the number of pregnant women with the disease may have been higher if the study had been conducted using different criteria. Some have criticised the definition of multimorbidity as simply having more than one disease, which would include a large population.24 In the future, a definition of multimorbidity that is suitable for the target community will be required since the significant diseases and conditions vary depending on the target population.24 Second, it was difficult in this study to discuss the biological mechanisms of the association between multimorbidity and neurodevelopmental delay. The association between various diseases and neurodevelopmental delays has been reported in previous studies.8 16–18 25 Further studies on disease characteristics and disease combinations may allow for hypotheses to be made regarding the biological mechanisms underlying the association between multimorbidity and neurodevelopmental delay. Third, as participants in the JECS were only collaborators, selection bias may have occurred.15 The prevalence of multimorbidity and the results of the association between multimorbidity and neurodevelopmental delay might have been different if the study design included pregnant women who did not participate in the JECS. The number of pregnant women with multimorbidities would increase and the results of the effects on the neurodevelopment of the children might be different if all pregnant women and children registered in the administration were included in the study. Fourth, we did not use the data on maternal situation after delivery. Incomplete questionnaire responses were reported to be influenced by the maternal situation after delivery as health status, number of siblings, partner and primary caregiver.26 27 The ASQ-3 scores of the offspring who were excluded were lower than those of the offspring included in most time points. In the analysed population, the changes in the ASQ-3 scores of the offspring tested less frequently differed from those of the offspring tested at all time points. Except for the group tested at all time points, the number of the offspring tested tended to decrease with age. It was difficult to examine the association between incomplete responses and the ASQ-3 scores in this study. In the future, we need to consider studies with regard to incomplete participants and neurodevelopmental delay of offspring. There was no analysis of data from offspring, such as birth weight, gestational age at birth, nutritional status and Apgar score, but, as we mentioned above, they were not selected as adjusted variables because we considered them as intermediate variables in the association between multimorbidity and neurodevelopmental delay.
Previous reports on multimorbidities in pregnant women have focused on its prevalence and impact on pregnant women themselves.5–7 This study is a new report in terms of the effect of multimorbidity in pregnant women on their offspring and provides important recommendations regarding the health of pregnant women.
Conclusion
This study demonstrated an association between multimorbidities in pregnant women and neurodevelopmental delays in their offspring in Japan. To clarify its mechanisms and effects, more research needs to be done in many regions of the world with different economic, geographic and racial conditions.
Data availability statement
Data may be obtained from a third party and are not publicly available. Data are unsuitable for public deposition due to ethical restrictions and legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amendment on 9 September 2015) to publicly deposit the data containing personal information. Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restrict the open sharing of the epidemiologic data. All inquiries about access to data should be sent to: jecs-en@nies.go.jp. The person responsible for handling enquiries sent to this e-mail address is Dr Shoji F. Nakayama, JECS Programme Office, National Institute for Environmental Studies.
Ethics statements
Patient consent for publication
Ethics approval
The JECS protocol was reviewed and approved by the Ministry of the Environment’s Institutional Review Board on Epidemiological Studies and the Ethics Committees of all participating institutions (Number 100910001). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We would like to express our gratitude to all the JECS study participants and staff members involved in data collection. We would like to thank Editage (www.editage.com) for the English language editing.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Collaborators Members of the JECS Group are as of 2023: Michihiro Kamijima (principal investigator, Nagoya City University, Nagoya, Japan); Shin Yamazaki (National Institute for Environmental Studies, Tsukuba, Japan); Yukihiro Ohya (National Center for Child Health and Development, Tokyo, Japan); Reiko Kishi (Hokkaido University, Sapporo, Japan); Nobuo Yaegashi (Tohoku University, Sendai, Japan); Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan); Chisato Mori (Chiba University, Chiba, Japan); Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Tomotaka Sobue (Osaka University, Suita, Japan), Masayuki Shima (Hyogo Medical University, Nishinomiya, Japan), Seiji Kageyama (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Shoichi Ohga (Kyushu University, Fukuoka, Japan), and Takahiko Katoh (Kumamoto University, Kumamoto, Japan).
Contributors TA and YaS designed this study. JECS collected the data and obtained funding. YaS, EY, KNag, ST, YI, CM, SI and RK collected the data. TA and YaS conducted the data analysis. TA, YaS, EY, YuS, KNak, YK, KNag, ST, YI, HI, TY, CM, SI, and RK contributed to data interpretation. TA, YaS, EY, YuS, KNak, YK, KNag, ST, YI, HI, TY, CM, SI, RK and the JECS Group conducted critical reviews. TA drafted the manuscript. YaS made critical revisions. YaS is responsible for the overall content as guarantor. All the authors have reviewed and commented on the manuscript. All the authors approved the final manuscript.
Funding This study was funded by the Ministry of the Environment, Japan. The findings and conclusions of this study are solely the responsibility of the authors and do not represent the official views of the government.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer-reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.