Article Text
Abstract
Objective To compare the efficacy and safety of different anti-vascular endothelial growth factor (VEGF) agents combined with different delivery methods for neovascular glaucoma (NVG).
Design Systematic review and Bayesian network meta-analysis (NMA).
Data sources PubMed, Embase, Cochrane Library, Web of Science, ClinicalTrials.gov, ISRCTN and Chinese databases including the China National Knowledge Infrastructure, China Science Periodical Database (Wanfang Database), VIP Journal Integration Platform and China Biology Medicine Database were searched from inception to 5 September 2022.
Eligibility criteria We included randomised controlled trials (RCTs) that investigated the treatment of NVG using different anti-VEGF agents combined with various methods of drug administration, without any language limitations. All patients included underwent panretinal laser photocoagulation and there were no restrictions on prior glaucoma surgery.
Data extraction and synthesis Two independent reviewers extracted data and assessed the risk of bias. Random-effect Bayesian NMA was conducted to compare the efficacy and safety and rank priority of anti-VEGF regimens. The source of heterogeneity and the related factors affecting the stability of the results were also explored. CINeMA (Confidence in Network Meta-Analysis) was used to assess the certainty of evidence.
Results Our analysis included 17 RCTs involving a total of 1311 eyes from 1228 patients. We examined five different treatment regimens, which used three different anti-VEGF drugs. The following treatments showed a significant decrease in intraocular pressure (IOP) compared with the control group at 1 month after glaucoma surgery: simultaneous intravitreal and intracameral injection of conbercept (ICCIVC) (mean difference (MD)=−11.56, 95% credible interval (CrI) −20.8 to −2.24), intravitreal injection of conbercept (MD=−8.88, 95% CrI −13.93 to −3.78), intravitreal injection of ranibizumab (MD=−7.62, 95% CrI −10.91 to −4.33) and intravitreal injection of bevacizumab IVB) (MD=−5.51, 95% CrI −10.79 to −0.35). The surface under the cumulative ranking curve (SUCRA) analysis indicated that ICCIVC (82.0%) may be the most effective regimen in reducing IOP. In terms of safety, there were no statistically significant differences among the interventions. According to the SUCRA analysis, ICCIVC (68.0%) was considered the safest choice with the fewest complications. Subgroup and meta-regression analyses showed that mean age was the main source of heterogeneity. Sensitivity analysis demonstrated the robustness of the study results.
Conclusion ICCIVC was more effective and safer than other anti-VEGF regimens for NVG. Simultaneous intravitreal and intracameral injection was found to be the best route of administration, and conbercept was found to be the superior drug selection when compared with ranibizumab and bevacizumab.
PROSPERO registration number CRD42022309676.
- Glaucoma
- Medical ophthalmology
- Ophthalmology
Data availability statement
Data sharing not applicable as no data sets generated and/or analysed for this study. Materials generated or analysed during this study are included in this published article and supplementary files.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
Network meta-analysis is the best method to compare interventions in the absence of head-to-head trials.
To the best of our knowledge, this study is the most comprehensive network meta-analysis conducted to date as it includes all available data from comparative studies.
Subgroup and meta-regression analyses were performed to examine the heterogeneity within the included studies.
Sensitivity analysis was additionally conducted to assess the impact of small sample sizes and significant heterogeneity on the study results.
Most of the included studies were conducted in Asia, and as a result conbercept was the most commonly used anti-vascular endothelial growth factor agent, which could potentially have introduced a selection bias that may have influenced the results.
Introduction
Neovascular glaucoma (NVG) is a secondary type of glaucoma that has the potential to cause vision loss. It occurs when abnormal new blood vessels form and obstruct the normal drainage of the aqueous humour in the eye.1 It is typically associated with ocular ischaemic diseases, such as diabetic retinopathy, central retinal vein occlusion and ocular ischaemic syndrome.2 Although NVG is a relatively rare condition, with a prevalence ranging from 0.01% to 0.12% in the population, it accounts for approximately 3.9% of all glaucoma cases and 9%–14.7% of all cases of secondary glaucoma.3
The treatment approach for NVG typically involves two main aspects: reducing vascular drive and controlling intraocular pressure (IOP).4 5 To address neovascularisation, common therapeutic options include panretinal photocoagulation (PRP) or the administration of vascular endothelial growth factor (VEGF) inhibitors. At the same time, effective control of IOP is vital to prevent damage to the optic nerve and is achieved through the use of topical and systemic medications or surgical interventions.
Initially used in ophthalmology for treatment of choroidal neovascularisation in age‐related macular degeneration, the application of anti‐VEGF medications has expanded rapidly to encompass the treatment of various other conditions.6 7 The currently available VEGF inhibitors, including bevacizumab (Avastin), ranibizumab (Lucentis), aflibercept (Eylea) and conbercept (Lumitin), have been proven to be effective in suppressing anterior segment neovascularisation and lowering IOP.3 8–10 These medications are administered via intravitreal, intracameral, and less frequently simultaneous intravitreal and intracameral routes for NVG treatment.11–14 Numerous researchers have also verified the effectiveness of these delivery modalities.15–17
We conducted a comparative analysis of different available anti-VEGF regimens (agents and delivery methods) for NVG using data obtained from randomised controlled trials (RCTs) in order to rank their priority, with the aim of guiding clinical practice.
Methods
This network meta-analysis (NMA) is reported following the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension statement for reporting NMAs.18 The protocol for this study has been registered with PROSPERO under registration number CRD42022309676.
Search strategy
Two authors independently searched PubMed, Embase, Cochrane Library, Web of Science, ClinicalTrials.gov, ISRCTN and Chinese databases including the China National Knowledge Infrastructure, China Science Periodical Database (Wanfang Database), VIP Journal Integration Platform and China Biology Medicine Database from database inception to 5 September 2022, with no language restrictions. The Chinese literature mainly selects high-quality studies such as the Chinese Medical Association or core journals. A detailed process is provided in online supplemental material 1.
Supplemental material
Inclusion and exclusion criteria
We included studies based on the following criteria: (1) participants: patients with a diagnosis of NVG; (2) interventions: anti-VEGF agents were administered in combination with diverse treatment regimens featuring different delivery modes—all eligible patients underwent PRP and there were no restrictions on prior glaucoma surgery; (3) comparators: placebo control, no-treatment control and positive control; (4) outcomes: the results of the included studies need to meet at least one outcome measure as defined in this NMA; and (5) study type: RCTs.
Studies that met any of the following criteria were excluded: (1) conference abstracts, reviews, meta-analyses or case reports; (2) patients with unknown or other types of glaucoma; (3) history of anti-VEGF or steroid injection, studies related to drug dosage, studies related to comparison of surgical methods and studies related to unplanned PRP; and (4) poor-quality Chinese studies.
Outcome measures
We took the IOP (mm Hg) at 1 month after antiglaucoma surgery (a: IOP 1 month) and the incidence of postoperative complications during the follow-up period (b: complications) as our primary efficacy and safety outcomes, respectively. Complications encompassed bleeding-associated complications such as hyphaema, vitreous haemorrhage or suprachoroidal haemorrhage.
The secondary efficacy outcomes included the success rate of antiglaucoma surgery (c: success rate), using the definitions by the authors of the individual studies; the visual retention rate after antiglaucoma surgery (d: visual retention rate), where visual retention was determined by improved or unchanged visual acuity; and IOP at 6 months after antiglaucoma surgery (e: IOP 6 months). In order to minimise bias, we preferably selected a common follow-up time point for the above outcomes. If a common time point was not available in the data, we used the available information during the follow-up period. Additionally, for controllable NVG cases which did not require glaucoma surgery, the IOP at 1 month after anti-VEGF treatment was evaluated (f: non-surgical IOP 1 month).
Study screening process
The selection of studies was independently conducted by two review authors to ensure reliability. Any discrepancies or disagreements were resolved through discussion between the two authors. Disagreements were resolved by a third review author.
Data extraction
Two authors independently extracted the following data: study characteristics (including randomisation method and masking of treatment allocation), patient characteristics (mean age, sex, primary disease, stage of NVG, baseline IOP, visual acuity), intervention measures (anti-VEGF drug types and administration methods, antiglaucoma surgery methods) and outcome variables.
Risk of bias assessment
Two authors used the revised Cochrane risk-of-bias tool for randomised trials (RoB2) to assess the risk of bias; disagreements were resolved through discussion with a third investigator.19
Statistical analysis
For outcomes with at least two direct comparative studies available, we conducted a pairwise random-effects meta-analysis using STATA (V.15.0). Categorical outcomes were assessed using ORs with corresponding 95% CIs, while continuous outcomes were evaluated using mean differences (MD) with 95% CIs.
Whenever the evidence formed a connected network diagram, a random-effect Bayesian NMA was conducted in OpenBUGS (V.3.2.3).20 We calculated the OR and the 95% credible interval (CrI) for categorical outcomes, along with MD and 95% CrI for continuous outcomes, to estimate the regimens’ effect size, respectively. The summarised estimates were calculated using Markov chain Monte Carlo (MCMC) methods.20 To estimate the posterior distribution for each model, three MCMC simulations were initialised using 200 000 iterations for each simulation. However, the results are reported after excluding the first 100 000 iterations. Convergence was assessed by visually inspecting history and trace plots.
For standard pairwise meta-analyses, we estimated heterogeneity variances for each direct comparison, which was conducted by I2, and the between-studies variance estimate obtained by τ2 (profile likelihood estimator).21 The heterogeneity variance, denoted by σ, represented the estimated SD between studies in the NMA for each outcome.22
We used the ‘design-by-treatment’ interaction method and the node-splitting method to examine global and local consistency, respectively.23 24 Additionally, we also used the node-splitting method to examine loop-closed inconsistency.25
The regimens were ranked based on the surface under the cumulative ranking curve (SUCRA).26 A higher SUCRA indicates better treatment efficacy.27 28 To summarise the efficacy and safety of all regimens, the resultant rankings are presented by clustered ranking plot.
Reporting bias assessment
We plotted the comparison-adjusted funnel plot to investigate small-study bias and the possibility of publication bias at the network level.29
Subgroup, meta-regression and sensitivity analyses
Subgroup and meta-regression analyses were conducted to explore the source of heterogeneity when there were more than 10 studies, or when the number of studies included in the analysis was greater than the number of treatments. Specifically, we investigated whether the surgical methods for antiglaucoma, proportion of retinal vein occlusion in the primary disease and mean age were significant sources of heterogeneity. We also performed sensitivity analyses to examine the robustness of our results. Specifically, we removed studies that fell outside the funnel plot, as well as small sample studies at the bottom of the funnel plot. These analyses helped us assess the impact of potential sources of bias on the overall results of our study.
Confidence in cumulative evidence
The overall quality of evidence was assessed by the Confidence in Network Meta-Analysis (CINeMA) approach.30 This method involves evaluating the quality of evidence for each outcome, considering factors such as risk of bias, inconsistency, indirectness, imprecision and publication bias. Based on the results of the evaluation, we downgraded the quality of evidence when appropriate and assigned a final confidence rating of high, moderate, low or very low.
Patient and public involvement
None.
Results
Literature search
The initial search of electronic databases and trial registration platforms yielded a total of 1112 records. After excluding 524 articles due to duplications and another 548 articles based on reading the titles and abstracts, we selected 40 potentially eligible citations for full-text review. After a careful review of these full-text articles, we excluded 23 reports, resulting in 17 trials that met our inclusion criteria. These 17 trials involved 1228 participants, with a total of 1311 eyes.31–47 An outline of the study selection process is shown in figure 1.
Flow chart of the study selection process. PRP, panretinal photocoagulation; RCT, randomised controlled trial.
Characteristics of the included studies
All 17 RCTs were two-arm studies. Of these, 13 studies involved antiglaucoma surgery and a total of 821 eyes. The remaining six studies, which involved 490 eyes, did not include antiglaucoma surgery. However, among them, there were two studies that included both antiglaucoma surgery and no antiglaucoma surgery groups.
Our studies covered a blank control group (Blank) and five different regimens for three anti-VEGF drugs, which are intravitreal injection of conbercept (IVC), intravitreal injection of ranibizumab (IVR), intravitreal injection of bevacizumab (IVB), intracameral injection of conbercept (ICC), and simultaneous intravitreal and intracameral injection of conbercept (ICCIVC). In total, there were 15 possible comparisons between these treatments. Of these, six comparisons were made directly in the included studies. The baseline characteristics of each study are presented in online supplemental material 2.
Supplemental material
Risk of bias results
The overall bias of the included RCTs was as follows: low risk 0%, some concerns risk 41.2% and high risk 58.8%. It is important to note that due to the severe clinical symptoms and complications associated with NVG, it is challenging to conduct completely double-blind studies in this field. This limitation often leads to a higher overall risk of bias in the included literature. The details of the risk of bias assessment are shown in online supplemental material 3.
Supplemental material
Pairwise meta-analysis
In terms of success rate, IVR (I2=0%, τ2=0, OR=0.25, 95% CI 0.10 to 0.68, p=0.006) was higher than Blank. Regarding IOP, we found that after 1 month both IVR (I2=99%, τ2=24.86, MD=7.28, 95% CI 2.83 to 11.74, p=0.001) and IVB (I2=76.4%, τ2=10.39, MD=5.37, 95% CI 0.87 to 9.88, p=0.019) were lower than Blank. After 6 months, IVR (I2=98.2%, τ2=41.28, MD=8.42, 95% CI 1.97 to 14.86, p=0.011) was lower than Blank. In the non-surgical IOP 1 month group, the effect of IVR (I2=99%, τ2=26.17, MD=13.54, 95% CI 6.41 to 20.66, p<0.001) was found to be more effective than Blank. However, these studies all have significant heterogeneity and no any statistically significant differences were found between treatment groups in terms of visual retention rate and complications. The results of the pairwise meta-analysis for each outcome are presented in online supplemental material 4.
Supplemental material
Network meta-analysis
Primary efficacy outcome: IOP at 1 month
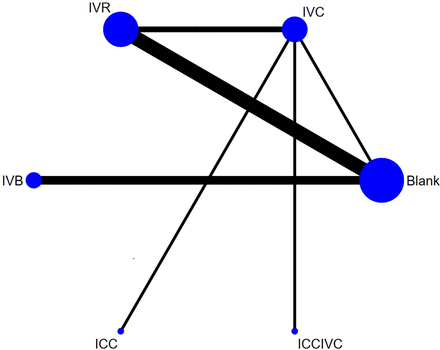
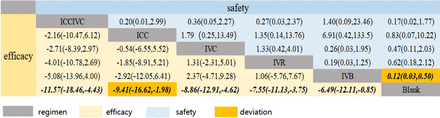
Thirteen studies involving 821 eyes and 6 regimens reported IOP 1 month after surgery, and there were 15 treatment comparisons. The network plot is shown in figure 2. Comparing the treatments with the Blank control, ICCIVC (MD=−11.56, 95% CrI −20.8 to −2.24), IVC (MD=−8.88, 95% CrI −13.93 to −3.78), IVR (MD=−7.62, 95% CrI −10.91 to −4.33) and IVB (MD=−5.51, 95% CrI −10.79 to −0.35) demonstrated a favourable effect on IOP 1 month after surgery. However, no statistical difference was found in the remaining comparisons (figure 3). ICCIVC had the highest rank (82.0%) in terms of efficacy in reducing IOP after 1 month. Following this, ICC (65.8%), IVC (64.4%), IVR (51.7%), IVB (35.0%) and finally the Blank control (1.1%) were ranked accordingly (online supplemental material 5A).
Supplemental material
Network plot of available treatment comparisons for the primary outcome. The size of the node represents the number of patients randomised to each regimen. Line width represents the number of randomised controlled trials comparing each pair of regimens directly. Blank, blank control group; ICC, intracameral injection of conbercept; ICCIVC, simultaneous intravitreal and intracameral injection of conbercept; IVB, intravitreal injection of bevacizumab; IVC, intravitreal injection of conbercept; IVR, intravitreal injection of ranibizumab.
Network meta-analysis of primary efficacy and safety outcomes. Regimens are reported in order of patients’ intraocular pressure 1 month after surgery ranking according to SUCRA. Blank, blank control group; ICC, intracameral injection of conbercept; ICCIVC, simultaneous intravitreal and intracameral injection of conbercept; IVB, intravitreal injection of bevacizumab; IVC, intravitreal injection of conbercept; IVR, intravitreal injection of ranibizumab; SUCRA, surface under the cumulative ranking curve.
The CINeMA assessment of the evidence in our study mostly rated the quality as very low. In the comparison-adjusted funnel plot (online supplemental material 5B), four studies were observed to fall outside the funnel plot, suggesting potential reporting bias, while one study was at the bottom of the funnel plot, indicating a small sample size. The meta-regression analysis revealed a significant association between effect size and mean age (0.68, 95% CrI 0.11 to 1.21). However, there was no association between effect size and the proportion of Retinal Vein Occlusion (RVO) (18.96, 95% CrI −12.38 to 50.04). Additionally, no statistical significance was found in the subgroup analysis between the different types of antiglaucoma surgery and effect size (2.44, 95% CrI −0.58 to 5.57). In the sensitivity analysis, we excluded the five studies that fell outside and at the bottom of the funnel plot and the results showed that the effectiveness of ICC (MD=−9.41, 95% CrI −16.62 to −1.98) was higher than Blank. However, the other regimens did not exhibit significant changes in their effectiveness (see figure 4 for details).
Sensitivity network meta-analyses for primary efficacy and safety outcomes. Regimens are reported in order of patients’ intraocular pressure 1 month after surgery ranking according to SUCRA. Blank, blank control group; ICC, intracameral injection of conbercept; ICCIVC, simultaneous intravitreal and intracameral injection of conbercept; IVB, intravitreal injection of bevacizumab; IVC, intravitreal injection of conbercept; IVR, intravitreal injection of ranibizumab; SUCRA, surface under the cumulative ranking curve.
Primary safety outcome: complications
Eleven studies involving 702 eyes and 6 different treatment regimens reported complications after surgery, leading to 15 treatment comparisons (figure 2). No significant differences were found in complications after surgery when considering all regimens (figure 3). With respect to ranking probabilities, ICCIVC ranked first (68.0%), followed by IVR (64.5%), IVC (58.2%), ICC (47.8%), IVB (35.4%) and Blank (26.0%) (online supplemental material 6A).
Supplemental material
Similar to the findings on efficacy, the CINeMA assessment indicated that the evidence quality for complications after surgery was mostly rated as very low. The comparison-adjusted funnel plot revealed that two studies fell outside the funnel plot, suggesting potential report bias and small sample size (online supplemental material 6B). The results of both meta-regression and subgroup analyses were consistent with the primary efficacy findings. Sensitivity analysis was performed by excluding two studies. After this exclusion, the remaining nine studies were subjected to NMA. The results indicated that, compared with the Blank control, IVB (OR=0.12, 95% CrI 0.03 to 0.50) was found to be safer. However, the other treatment regimens did not exhibit significant changes in terms of safety (figure 4).
Secondary efficacy outcomes: success rate
Seven studies involving 417 eyes and 4 regimens reported success rate after surgery, leading to 6 treatment comparisons. No significant differences were found between the treatment regimens in terms of success rate. According to ranking probabilities based on SUCRA, IVC ranked first (74.8%), followed by IVR (63.3%), IVB (59.1%) and Blank (2.8%). CINeMA assessment mostly rated the quality as very low (online supplemental material 7).
Supplemental material
Secondary efficacy outcomes: visual retention rate
Six studies involving 331 eyes and 5 regimens reported visual retention rate after surgery, resulting in 10 treatment comparisons. Due to the inability of the Bayesian methods to converge, we used a random-effects NMA within a frequentist framework, specifically using STATA (V.15.0). No significant differences were found between the treatment regimens. According to ranking probabilities based on SUCRA, IVC ranked first (94.1%), followed by ICCIVC (77.5%), Blank (43.1%), IVR (22.5%) and IVB (12.8%). CINeMA assessment mostly rated the quality as very low (online supplemental material 8).
Supplemental material
Secondary efficacy outcomes: IOP at 6 months
Nine studies involving 549 eyes and 5 regimens reported IOP 6 months after surgery, leading to 10 treatment comparisons. When compared with Blank, IVC (MD=−8.94, 95% CrI −15.8 to −2.08) and IVR (MD=−8.37, 95% CrI −12.42 to −4.35) exhibited significantly lower IOP 6 months after surgery. However, no statistical difference was found in the remainder of the treatment comparisons. According to ranking probabilities based on SUCRA, ICC ranked first (77.9%), followed by IVC (72.2%), IVR (67.9%), IVB (24.1%) and Blank (8.0%). CINeMA assessment largely rated the quality as very low (online supplemental material 9).
Supplemental material
Secondary outcomes: non-surgical IOP at 1 month
Six studies involving 490 eyes and 6 regimens reported non-surgical IOP 1 month after treatment, resulting in 15 treatment comparisons. When compared with the Blank, IVR (MD=−13.5, 95% CrI −18.98 to −8.03) showed a significantly lower IOP. However, no statistical difference was found in the remaining comparisons. According to ranking probabilities based on SUCRA, IVR ranked first (91.7%), followed by ICCIVC (67.9%), ICC (58.0%), IVC (44.0%), IVB (23.6%) and Blank (14.9%). CINeMA assessment mostly rated the quality as very low (online supplemental material 10).
Supplemental material
Efficacy versus safety in network analysis
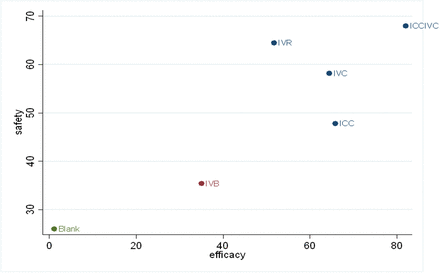
The clustered ranking plot, which compares the results of the primary efficacy and safety analysis, indicated that ICCIVC was the most efficacious and safest regimen. This was shown by the position of this regimen on the upper right corner of the plot in figure 5.
Clustered ranking plot of anti-vascular endothelial growth factor regimens for neovascular glaucoma based on primary efficacy and safety outcomes. Each colour represents a group of regimens that belong to the same cluster. Regimens lying on the upper right corner are the most efficacious and safest. Blank, blank control group; ICC, intracameral injection of conbercept; ICCIVC, simultaneous intravitreal and intracameral injection of conbercept; IVB, intravitreal injection of bevacizumab; IVC, intravitreal injection of conbercept; IVR, intravitreal injection of ranibizumab.
Inconsistency
Heterogeneity, as represented by SD (σ), was estimated at 3.77 (95% CI 2.441 to 4.918) for IOP at 1 month and 3.16 (95% CI 1.443 to 4.869) for complications. The test of global and local inconsistency did not detect any evidence of statistically significant inconsistency for the primary and secondary outcomes (global inconsistency: p=0.15–0.79). Among six outcomes, three outcomes covered loop-closed, all of which showed no significant inconsistency.
Discussion
To our knowledge, this study is the first to analyse the efficacy and safety of different anti-VEGF drugs combined with different delivery methods for NVG using Bayesian NMA and to prioritise different anti-VEGF regimens. At present, two NMAs on NVG can be retrieved, Dong et al’s48 49 results in 2018 and Lin et al’s study in 2022, which only compared the clinical efficacy and safety of various surgical interventions for NVG. However, combined with a large number of literature research and clinical evidence, it was found that different anti-VEGF drugs and their different routes of administration for the treatment of NVG also have differences in clinical efficacy and safety. For studies on anti-VEGF drugs in the treatment of NVG, Simha et al’s1 review in 2020, including four RCTs, indicated that the use of anti-VEGF drugs in patients with NVG resulted in better resolution of iris neovascularisation in the short term; however, the long-term benefits have not been concluded and there was insufficient evidence to assess the difference in adverse events with or without anti-VEGF drugs. A meta-analysis by Hwang and Lee50 in 2021 showed that the success rate of Ahmed glaucoma valve (AGV) +IVB treatment was higher than that of AGV treatment alone.50 The above studies only prove that the combination of anti-VEGF injections can produce positive impact on NVG; however, the studies did not analyse the efficacy of NVG treatment according to the different types of anti-VEGF and the different routes of administration.
In recent years, there has been an increase in the number of RCTs investigating the use of anti-VEGF drugs for NVG. However, some anti-VEGF therapies often lack head-to-head studies, which makes it difficult to directly compare their effectiveness. This study provides indirect comparative evidence through the transmission of NMA, and the results obtained from direct and indirect evidence were compared.
A total of 17 RCTs involving 1311 eyes of 1228 patients were included in this study. Three anti-VEGF drugs were analysed, involving five treatment regimens, which were ICCIVC, IVC, ICC, IVB and IVR.
Analysis of the primary efficacy outcome (IOP at 1 month) showed that ICCIVC, IVC, IVR and IVB were significantly more effective than Blank. Direct controlled studies were available to compare IVC, IVR, IVB and Blank. Because there were no direct controlled studies comparing ICCIVC and Blank, the evidence supporting this comparison came from indirect comparisons. Using SUCRA value as the effect size, cluster analysis suggested that ICCIVC had the most significant effect, followed by ICC, IVC, IVR and IVB. From the above ranking, it can be seen that conbercept has superior efficacy compared with ranibizumab and bevacizumab. Recent controlled clinical studies have also shown that conbercept has more advantages than ranibizumab in controlling IOP and improving visual acuity and has fewer postoperative complications. The analysis suggested that conbercept, formed by fusion of partial immunoglobulin regions of VEGF receptor-1 and VEGF receptor-2 with Fc fragment of human immunoglobulin G1, had a higher affinity for VEGF-A and placental growth factor (PIGF) compared with ranibizumab and bevacizumab.51 Considering the different delivery routes for conbercept, the analysis suggested that combined injection yields the best treatment effect, followed by intracameral injection, which is superior to intravitreal injection. Bhagat et al52 reported that the intracameral injection route was more effective in controlling IOP, possibly because the drug can directly reach the neovascularised blood vessels of the iris and chamber angle after the intracameral injection. Moreover, the local concentration of anti-VEGF drugs in the anterior chamber was higher with intracameral injection compared with intravitreal injection.
Analysis of the primary safety outcome (complications) showed no statistical difference among all interventions. From the perspective of cumulative ranking probability, ICCIVC may be the safest among the anti-VEGF regimens analysed, but its SUCRA value was only slightly higher than that of IVR. The safety SUCRA values for all five anti-VEGF regimens were not significantly different from Blank, which indicated that the effect of anti-VEGF injection on reducing postoperative complications was not very significant. A retrospective study demonstrated no significant correlation between IVB and hyphaema after antiglaucoma surgery,53 which is consistent with our results. Sugimoto et al54 believed that injection of anti-VEGF only reduces the neovascularisation on the surface of the iris, but may not completely eliminate the neovascularisation in the interstitium of the iris, which may explain why there was no significant effect on postoperative haemorrhagic complications.
The study conducted a reporting bias assessment for both the primary efficacy and safety outcome. It revealed the presence of publication bias and small sample size effects in both groups. Sensitivity analysis was carried out by eliminating the studies with large heterogeneity outside the funnel plot and the studies with small sample at the bottom of the funnel plot. The results did not change significantly, indicating that the results for the primary outcome were stable. In order to explore the heterogeneity of efficacy and safety, subgroup and meta-regression analyses were conducted, respectively, and the results showed that the mean age of the participants had an influence on the effect size of both groups.
In this study, only three anti-VEGF regimens were included in the success rate group. According to the SUCRA value, IVC had the highest ranking, followed by IVR and IVB. This ranking is consistent with the efficacy results observed in the primary outcome analysis, indicating that conbercept is of higher priority compared with ranibizumab and bevacizumab. It is important to note that the criterion for determining postoperative success in this outcome could not be uniformly established and had to be evaluated based on the definition used in each individual study. This variation in defining success may introduce some degree of bias in the analysis.
The IOP 6 months group represents the long-term efficacy of anti-VEGF for NVG. The results showed that IVC and IVR were significantly different from Blank. These findings for IVR were consistent with previous pairwise meta-analyses. However, the evidence for IVC was obtained through indirect comparison. ICC ranked highest according to SUCRA, followed by IVC, IVR and IVB. These rankings were similar to the efficacy results obtained in the primary outcome analysis. This indicates that conbercept demonstrated superior efficacy compared with ranibizumab and bevacizumab for long-term IOP control in patients with NVG.
For the non-surgical group, the analysis revealed that IVR was significantly more effective than Blank. According to the priority SUCRA value, the best treatment was IVR, followed by ICCIVC. However, previous studies have confirmed that intracameral combined with intravitreal injection can lead to rapid regression of iris and chamber angle neovascularisation, with a shorter regression time than intracameral or intravitreal injection alone.55 56 Therefore, the rank is different from the published studies. We speculate the following possible reasons for these differences: a total of six RCTs were included in this outcome index, three of which had a baseline average IOP of >40 mm Hg, representing uncontrolled NVG. According to treatment guidelines, antiglaucoma surgery should be considered for such cases; however, in the original studies, antiglaucoma surgery was not performed, and in this NMA they were included in the non-surgical group, which could have biased the results to some extent.
This study offers several notable advantages. First, it is the first study to comprehensively analyse the efficacy and safety of different anti-VEGF drugs in combination with different injection methods in the treatment of NVG. This allows for a comparison and prioritisation of different anti-VEGF regimens, providing valuable insights for clinical decision-making. Moreover, the study employed NMA, which is an optimal approach when direct head-to-head intervention analyses are lacking. To address heterogeneity in the primary outcome, the study conducted subgroup and meta-regression analyses. These analyses help investigate potential sources of variation and explore how factors like mean age may influence treatment outcomes. Additionally, sensitivity analysis was employed to assess the impact of small sample sizes and large heterogeneity on the results. This analysis helps evaluate the stability and consistency of the findings, even when considering potentially influential studies. Overall, the methodological approaches used in this study, including NMA, subgroup analysis, meta-regression analysis and sensitivity analysis, contribute to a comprehensive and well-rounded analysis of the efficacy and safety of different anti-VEGF drugs and injection methods for NVG treatment.
However, the results of the NMA should be interpreted with caution due to the following limitations: (1) As NVG is a rare disease, there is a lack of large-scale, multicentre RCTs, leading to a small sample size and limited number of included studies. (2) In this NMA, the credibility evaluation of CINeMA was generally low or very low, and the quality evaluation of the included studies was not ideal; the report bias identified through funnel plots suggested that publication bias may exist. (3) Most of the included studies were conducted in Asia, resulting in a higher prevalence of conbercept use, which could potentially introduce partial selection bias on the results. (4) For the analysis of complications, success rate and vision retention rate, the absence of definite time point data meant that data close to the specified time point were selected, leading to potential bias. In terms of success rate, the study used the original authors’ definition of surgical success criteria, which may have introduced bias and made direct comparison between studies difficult.
In summary, future research efforts should focus on conducting high-quality, large-scale, multicentre clinical RCTs that encompass a wider range of anti-VEGF drug regimens, thus generating more robust evidence to inform clinical practice and improve outcomes for patients with NVG.
Conclusion
This NMA provides substantial evidence for the clinical application of anti-VEGF drug regimens for NVG. Our findings suggest that ICCIVC is more effective and safer than the other four interventions included in the analysis. Simultaneous intravitreal and intracameral injection is the preferred route of administration. With regard to selecting a specific drug, conbercept is recommended over ranibizumab and bevacizumab.
Supplemental material
Data availability statement
Data sharing not applicable as no data sets generated and/or analysed for this study. Materials generated or analysed during this study are included in this published article and supplementary files.
Ethics statements
Patient consent for publication
Acknowledgments
We deeply appreciate all the researchers who performed the eligible studies that have been included in the present network meta-analysis.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors JWa conceived the study, drafted the protocol, collected the data, performed the statistical analysis and wrote the manuscript. Y-MG participated in data extraction and data analysis. Y-MG, JWe and JM contributed to the assembly of data, quality assessment and data interpretation. LY revised the manuscript, acquired funding, accepted full responsibility for the work of the study, had access to the data and controlled the decision to publish as the guarantor. All authors have read and approved the final version of the manuscript.
Funding This work was supported by Xi’an Medical Research-Discipline Capacity Building Project (project name: Genetic Mechanism of Early-onset High Myopia Based on Next-Generation Sequencing Technology; project no: 23YXYJ0002).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.