Article Text
Abstract
Objective This study aimed to assess the feasibility of a future trial comparing the collaborative care model with usual care for patients with musculoskeletal conditions and co-existing symptoms of anxiety and depression.
Design A single-centre, parallel-arm, one-to-one, randomised controlled trial design using a mixed-methods approach was used. semistructured interviews and focus groups were conducted post intervention with all participants and staff respectively to explore acceptability towards the model and identify recommendations for improvements.
Setting An orthopaedic rehabilitation outpatient tertiary hospital.
Participants Adult patients with musculoskeletal conditions and co-existing moderate or severe symptoms of anxiety and depression attending outpatient therapy appointments.
Intervention The collaborative care model consisted of a tailored management programme to facilitate the integration of care provided by physical and mental healthcare professionals. A case manager screened and coordinated targeted mental health support for participants. Participants allocated to usual care had no support from the case manager.
Main outcomes measure Feasibility indicators (rates of recruitment, randomisation and retention), acceptability of clinical outcome measures, usage of additional resources and cost of intervention implementation.
Results Of the 89 patients who provided consent to take part, 40 participants who matched the eligibility criteria were randomised to either the intervention (n=20) or usual care arm (n=20). Overall adherence to the intervention was 58.82%, while the withdrawal rate was 37.5% at 6 months. All of the 27 participants who were retained completed self-reported outcomes. Qualitative data highlighted that integrated mental health support was favourably perceived. In addition to prenegotiating protected psychology time, the need for operationalised communication between the case manager and clinicians was identified as a recommendation for a future trial.
Conclusions The trial and intervention were acceptable to patients and healthcare professionals. While the findings demonstrate the feasibility of trial recruitment, a future trial will require optimised retention strategies to improve adherence and withdrawal rates.
Trial registration number NCT05018039.
- Anxiety disorders
- Musculoskeletal disorders
- Depression & mood disorders
- MENTAL HEALTH
- Physical Therapy Modalities
- Randomized Controlled Trial
Data availability statement
Data are available on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Anxiety disorders
- Musculoskeletal disorders
- Depression & mood disorders
- MENTAL HEALTH
- Physical Therapy Modalities
- Randomized Controlled Trial
STRENGTHS AND LIMITATIONS OF THIS STUDY
The study followed a preregistered protocol to ensure transparency and minimise bias in the research process.
A mixed-method approach provided a holistic view of trial barriers and facilitators from varied perspectives.
Interviews and focus groups facilitated comprehensive insights from patients and healthcare professionals, complementing objective data.
The study was conducted at a single centre, limiting the generalisability of the results to broader healthcare settings.
Due to the nature of the intervention, blinding of healthcare professionals and participants was not possible, potentially introducing an element of bias into the study results.
Introduction
Musculoskeletal (MSK) conditions are the leading cause of disability worldwide, affecting approximately 1.71 billion people.1 In the UK, 17.8 million people are currently affected by MSK chronic conditions,2 where one in five adults consult their general practitioner (GP) regarding MSK symptoms each year.3 Chronic MSK conditions have been associated with approximately 30.8 million working days lost to absence, and a reduced ability to engage in social roles.2 On an individual level, these conditions can substantially affect aspects of quality of life, such as self-care, functioning and mental health.1 2 4
The interplay between physical and mental health has become increasingly acknowledged in recent years, as epidemiological evidence suggests that mental health conditions increase the chances of developing physical conditions.5 In the UK, one in six adults currently has a mental health condition such as anxiety and depression6 where the prevalence of self-reported mental health conditions is higher among people with MSK conditions, compared with those without (OR 1.4).7 For patients with both physical and mental health conditions in the orthopaedic setting, there is a greater risk of poor clinical outcomes, reduced patient satisfaction4 8 and increased needs for both patients and healthcare services.4
Mounting evidence supports the biopsychosocial approach to enhance clinical outcomes and quality of life,4 8 9 where integrated healthcare models, which facilitate effective management of both physical and mental health conditions have gained widespread acceptance.9–11 Previous systematic reviews have focused on psychological interventions such as cognitive–behavioural therapy in the management of MSK conditions such as back pain.10 11 However, there is limited evidence surrounding the people living with long term MSK conditions and mental health conditions.
Liaison psychiatry already plays an important role in hospital settings to assess and manage co-occurring mental health disorders.12 However, this approach traditionally operates on referral-and-triage, that is, a reactive approach.12 A potential proactive approach to facilitate the integration of physical and mental healthcare is through the implementation of the ‘collaborative care model’ (CCM). Collaborative care was initially developed in the 1990s in the USA to facilitate multidisciplinary working between physicians, psychiatrists and clinical care coordinators (case managers)13 and has since generated worldwide interest for its clinical and cost-effectiveness.13 The case manager is central to facilitating the integration of care provided by psychological and physical healthcare professionals through screening, systematic follow-ups and timely provision of care. Findings from randomised controlled trial (RCT) and a systematic review have shown that the implementation of a CCM enhances liaison psychiatry provision with a positive impact on clinical outcomes in specialist physical settings, such as renal care,12 diabetes,14 and oncology and chronic pain.15
Although the CCM has not yet found its place in clinical practice in the UK,13 the National Institute for Health and Care Excellence (NICE)16 recommends CCM implementation for people with moderate-to-severe depression and coexisting cancer and diabetes.14 15 To our knowledge, only one cluster RCT has investigated the effectiveness of Collaborative Care intervention for managing depression and chronic MSK pain in primary care.17 This study revealed significant improvements in depression severity after 12 months for patients under the collaborative care arm. However, pain levels remained unchanged due to a ‘low intensity’ intervention design and inadequate adherence by both patients and physicians. Furthermore, no qualitative evaluation explored the potential reasons contributing to low adherence.
Before a multicentre RCT can test CCM’s clinical and cost-effectiveness, feasibility and acceptability must be explored in accordance with the MRC guidelines for developing and evaluating complex interventions.18 The primary aim of this study was to determine the feasibility and acceptability of conducting a future RCT evaluating the clinical and cost-effectiveness of the CCM for people with MSK and coexisting mental health conditions.
Methods
Study design
This study followed a preregistered protocol,19 where a single-centre, parallel-arm, non-blinded RCT design using a mixed-method approach was implemented between February 2022 and October 2022. Participants were required to remain in the trial for a total of 6 months. Qualitative data were collected between October 2022 and December 2022. The Consolidated Standards of Reporting Trials checklist for pilot or feasibility trial20 was used.
Setting and participants
The trial was conducted in a tertiary National Health Service (NHS) hospital specialising in orthopaedic conditions, ‘in the’ UK. Healthcare professionals were briefed on the eligibility criteria (table 1) and introduced the study during initial appointments. Interested participants could meet a research team member postappointment for further details about the study.
Inclusion and exclusion criteria
Screening and enrolment
Patients gave written consent within 3 weeks of initial contact during MSK appointments. The principal investigator screened them with the Patient Health Questionnaire (PHQ)-Anxiety and Depression Scale (ADS) questionnaire, communicating reasons for exclusion (online supplemental file 1).
Supplemental material
Randomisation and blinding
Of the 89 patients who provided consent to take part, 40 participants who matched the eligibility criteria were randomised according to a 1:1 ratio usual care (n=20), CCM (n=20). Allocations were concealed and undertaken via online randomisation software (https://www.sealedenvelope.com/)21 by the principal investigator. Given the focus on evaluating the feasibility of providing case manager support (the CCM), blinding healthcare professionals or participants was not possible.
Intervention: CCM
The CCM intervention involved the provision of tailored mental and physical healthcare (delivered by physiotherapists, occupational therapists, psychiatrists and psychologists), which was coordinated through the support of a designated case manager. The case manager (who was an assistant psychologist) organised necessary mental health support according to individual needs that were identified through initial screening procedures. Following screening, the remit of the case manager was to:
Develop personalised care plans.
Co-ordinate psychological and MSK outpatient appointments.
Monitor progress (through validated clinical questionnaires) and adjust support/appointments as necessary.
Streamline communication between physical and mental healthcare providers, as well and maintaining contact with the participant.
The provision of the case manger support was delivered in additional to the routine physiotherapy/occupational therapy outpatients’ appointments (usual care) through in-person, phone or video consultations on a monthly basis, but weekly contacts were needed at times.
Usual care
Physiotherapists or occupational therapists assessed participants’ needs, creating personalised rehabilitation plans. Therapy included 1:1 sessions and potential group classes. Physiotherapy featured exercises and education, while occupational therapy addressed activities of daily living. Additional mental health support was sought through GP referrals or internal Trust mental health services, if required, following standard care procedures.
Data collection
All participants from both arms of the trial were asked to complete four baseline questionnaires after randomisation, PHQ-ADS,22 EuroQol-5 Dimension-3 Level (EQ-5D-3L),23 MSK Health Questionnaire (MSK-HQ),24 Numerical Pain Rating Scale (NPRS)25 and the Pain Disability Index (PDI),26 which took up to 25 min to complete. These included tailored questionnaires on demographic data (age, ethnicity, marital status, highest qualification level and employment status), medical history, current medication usage and self-reported measures.
Participants repeated baseline self-reported outcome measures at the 6-month follow-up, reported medication changes and indicated their progress through the Global Rating of Change (GROC).27 Usage of healthcare resources was documented, collected through face-to-face, phone or video appointments based on participant choice and availability.
Primary outcomes
The feasibility outcomes were participation, randomisation, retention and adherence to the intervention at month 6. Some criteria for progression were established: minimum consent rate of 20%, minimum recruitment rate of 10%, maximum withdrawal rate 25% and minimum adherence rate of 75%.
Participation and randomisation
This feasibility trial used descriptive analyses without hypothesis testing, hence no formal sample size calculation was performed. The goal was to recruit 40 patients in 3 months, estimating a recruitment rate within ±6% at a 95% confidence level.
Retention and adherence
Retention was calculated as participants who remained in the study at month 6, while adherence was the percentage of attended appointments out of the total number of booked appointments.
Secondary outcomes
Secondary outcomes aligned with testing the intervention and its real-world implementation.27 These outcomes included acceptability of self-reported measures, trial acceptability for patients and professionals (including barriers and facilitators), additional healthcare resource usage and staff costs estimation for intervention arm.
Acceptability of self-reported measures
Various Patient-Reported Outcome Measures (PROMs) were collected, with necessary copyrights obtained. These focused on anxiety, depression, quality of life, physical health, pain and global change.
Anxiety and depression
The 16-item PHQ-ADS22 was used to measure the severity of anxiety and depressive levels.
Quality of life
The five-item EQ-5D-3L23 is a standardised measure for health-related quality of life, recommended by NICE16 for clinical trial economic evaluations.
Quality of physical health
The 14-item MSK-HQ24 assesses several domains: pain severity, physical function, work interference, social interference, sleep, fatigue, emotional health, physical activity, independence, understanding, confidence to self-manage and overall impact.
Level of pain
Two measures were used to assess overall pain levels, namely the 11-point NPRS25 and the PDI.26 PDI assesses the impact of chronic pain on patients’ daily lives and measures seven life activity categories. NPRS scoring was from 0 (no pain) to 10 (worse).
Global change
The 15-item GROC27 scale can indicate whether an overall condition is improving or worsening, as well as indicate the extent of this change.
Acceptability of the trial by patients and healthcare professionals
Participant feedback was evaluated through a patient-centred approach.28 This involved interviews with patients and focus groups with healthcare professionals that were facilitated by the principal investigator who is an expert qualitative methodologist and did not have prior participant contact. Interview and focus group guides were prepared by the research team (online supplemental files 2 and 3). All participants from both arms were invited to participate in interviews within a month of completing the 6-month follow-up either face to face, via telephone or through video call. Furthermore, 20 healthcare professionals involved in participant care were invited to join two virtual focus groups via teams, within 4 weeks after the trial completion.
Supplemental material
Supplemental material
Additional healthcare resources
Establishing whether additional healthcare resources could be estimated by participant self-report form.
Staff costs and main resources to implement the CCM
Staff costs and resources for the intervention arm were estimated based on the number, type and duration of appointments conducted by the case manager, therapists and mental health specialist. Data were collected from the hospital therapies appointment booking system and the case manager’s diary.
Data analysis
Data collected during this study will be made available on request from the corresponding author, if appropriate. The data will not be made publicly available in accordance with General Data Protection Regulation.
Quantitative data analysis
This trial primarily focused on assessing the feasibility of a future RCT, involving a descriptive analysis of key process-related outcomes. Quantitative data were analysed using SPSS (version 22.0),29 with recruitment and retention measured by absolute and relative frequencies. Healthcare resource utilisation was described by type and frequency. Clinical outcomes’ acceptability was stated as completion percentages. The statistical analysis plan was planned by the study statistician. Staff costs for CCM participants’ care were calculated using the National Cost Index (NCI),30 except for the case manager’s hourly cost, as their role was outside the NCI scope.
Qualitative data analysis
Interviews and focus groups were transcribed by an external company, then checked by the principal investigator and imported into NVivo V.12.31 Two research team members independently analysed participant and healthcare professional transcripts, resolving discrepancies with a third member to establish coding consensus. Analysis, using the ‘normalisation process theory’,32 began soon after data collection began.
Suicidal ideation and risk of self-harm protocol
For suicide risk, we implemented the Columbia-Suicide Severity Rating Scale33 protocol with a created flowchart for follow-up actions (online supplemental file 4). A steering committee supervised the trial.
Supplemental material
Patient and public involvement
Patient stakeholders played a vital role in shaping the study’s design, impacting its duration and reducing patient burden. Self-assessment measures were thoughtfully chosen to characterise this specific population. Three patients significantly contributed to creating patient materials and consent forms. Another three patients actively participated in the steering committee, attending meetings to address emerging issues and ensure the study’s smooth operation.
Results
Baseline characteristics
Participant characteristics were mostly well balanced between the two groups at baseline. The average age of participants in the intervention and usual care arm was 48.5 (±15.9) and 473 (±181) years, respectively, where there were more women than men in both groups. The ethnicity of participants under both arms was mostly English, while more participants under the intervention arm had a spouse/partner (n=12 vs n=7). Demographics are presented in table 2.
Patient demographic
Feasibility
Participation and randomisation
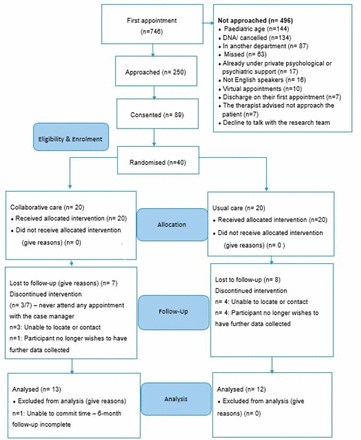
A total of 250 patients were approached and invited to participate during the study recruitment period between January 2022 and May 2022. Eighty-nine (35.6%) of the 250 patients provided consent between February 2022 and May 2022, where 40 of whom were deemed eligible for the trial following the screening process. These 40 participants were subsequently randomised to either the usual care arm (n=20) or the intervention arm (n=20) (see figure 1).
CONSORT flow chart. CONSORT, Consolidated Standards of Reporting Trials.
At baseline, 20 participants (50%) presented with moderate levels of anxiety and depression, while the other 50% reported severe levels. Regarding the risk of suicide, 19 (47.5%) of the 40 participants randomised had risk of suicide: 13 (68.4%) had low risk, 4 (21.1%) had moderate risk and 2 (10.5%) high risk according to CSSR-S. On month 6, of the 25 participants retained, 8 (24%) presented mild levels of anxiety and depression; 10 (40%) moderate levels and 9 (36%) with severe levels. Only one participant presented a low risk of suicide.
Retention and adherence
Twenty-five participants from the intervention and usual care arm were retained until the final follow-up at 6 months. The overall withdrawal rate was 37.5%, which was higher than the 25% threshold specified within the success criteria (table 3). Nevertheless, retention was similar among both groups, that is, usual care n=8, intervention n=7. Reasons for non-attendance can be found in figure 1. A total of 102 appointments were booked for participants under the intervention arm by the case manager. Sixty out of the 102 appointments were attended by 17 participants. The average adherence rate for participants under the intervention arm was 58.8%. Monthly variations in adherence rates were observed. Month 4 had the lowest adherence rate (35.3%), while month 6 had the highest (76.4%). All self-reported measures (100%) were completed at baseline and 6-month follow-ups for the 25 retained participants, with only 3 missing data points.
Feasibility outcomes summary
Secondary outcomes
All intervention and usual care participants were interviewed. A total of 25 participants participated in interviews, and 8 of the 20 healthcare professionals joined a focus group.
Acceptability of the trial by patients
Twenty-five participants (intervention arm: n=13, usual care arm: n=12) consented to interviews. Both groups acknowledged the trial’s importance in their care, valuing the inclusion of mental health alongside physical health. The case manager was a central figure, appreciated by most in the intervention arm, although one participant had higher expectations for their involvement. One participant expected the case manager to track investigations and appointments closely. Patients highlighted that this type of intervention should happen earlier in their care additional information can be found in online supplemental file 5.
Supplemental material
I think it was really positive. I think you've got the right people. I think the message is very clear. That there is a link between your physical illness and your mental illness. To be able to link the two and understand has been very positive. Participant 66, Collaborative Care
One participant reinforced that the trial changed her life.
I think it’s made …. life changing for me, really, because as I say, it was - it’s gone from me and you having a chat to being able to sit down with [case manager] and get my problems out in the open and talk about them. Then I'm getting a psychiatrist who’s helping me with my pain and dealing with that, so that’s a massive benefit for me. Then dealing with my psychological problems as well, I've spoken about them, about what my issues are been given some tools to maybe help, to start me off with, before I get a proper appointment. Participant 31, Collaborative Care
Facilitators and barriers
All 25 participants valued the trial, citing benefits like being heard, access to psychiatric support and reduced risk of suicide for two participants (one from usual care arm), emphasising trial significance.
I think it’s important. It’s imperative, but it doesn’t happen. Mental health is so important but it’s so—the service is so overrun at the moment that it’s impossible to access anything. Participant 82, Usual Care
Participants reported challenges, such as having to allocate personal time for case manager appointments and experiencing emotional discomfort during the trial.
Acceptability of self-reported measures
In interviews, 20 patient participants (intervention=11, usual care=9) provided feedback on questionnaires, highlighting areas for improvement such as clarity, simpler language and shorter formats. Some found certain questionnaires overly generic and potentially dehumanising. Concerns arose about sensitive topics like suicide, pain and depression. The summary of PROMs acceptance is in online supplemental file 6. Two participants suggested including open-ended questions in future questionnaires to allow patients to express their opinions and feelings during clinical care.
Supplemental material
So, I don’t know, maybe if they were changed, perhaps there should be a section where you can actually have a comment perhaps so that it isn’t as cut and dried. Participant 75, Collaborative Care
Acceptability of the trial by clinicians: focus group
Eight (40%) out of 20 potential healthcare professionals under the intervention and usual care arm participated in the focus groups who had patients allocated to both arms. Baseline characteristics are presented in table 4. Some quotations from focus groups’ findings are presented in online supplemental file 7.
Supplemental material
Clinicians’ demographic
Overall, healthcare professionals largely viewed the trial as positive. They cited the significance for integrating physical and mental healthcare in an MSK context, addressing holistic patient needs and the importance of anxiety and depression risk scores in their practice. The suicide flow chart was praised for guiding referral pathways and formal mental health training was welcomed. Professionals noted limited mental health resources, highlighting the trial’s potential for improving communication among all involved in patient care.
I think it felt really good that it was being recognised that it’s not just a physical presentation of a condition that we’re able to look at the whole person. Participant 5
Clinicians expressed a preference for the Case Manager to offer more comprehensive patient information and engage in formal meetings. They were aware of patients at suicide risk but lacked updates on their care progress or referrals to mental health services.
Probably, similarly. I mean, I just had contact with her [case manager] kind of discussing patients and saying, yes, this patient is happy to chat to you, and all of those interactions were quite positive. But that was probably the extent of it for me. Participant 8
Additional healthcare resources
Participants in both the intervention and usual care arms accessed a similar number of additional healthcare resources beyond their regular appointments. Specifically, in the intervention arm, 13 participants accessed a total of 51 appointments (29 with their GP, 15 with a private physiotherapist, 5 Accident and Emergency (A&E) visits, 1 with an osteopath and 1 with a private psychologist). In comparison, the 12 participants in the usual care arm accessed 50 appointments (21 with their GP, 10 with a private physiotherapist, 7 with a chiropractor, 6 gym sessions, 4 A&E visits, 1 obstetric appointment during the trial, 1 with an NHS psychologist outside of the Trust and 1 with a private psychiatrist)
Staff costs required to deliver the intervention
Thirteen participants in the intervention arm contributed to cost calculations. Case manager appointments included 62 sessions (30 face to face, 19 via teams, 13 by phone) totalling 65 hours and £1120.6. Booking/rescheduling time was not included. Physiotherapist appointments comprised 34 sessions (13 first, 21 follow-ups), totalling 23.5 hours and £427.46, however, occupational therapy was not accessed. Psychiatrist appointments (5, 1 hour each) cost £1856.5. Seven participants had clinical psychologist triage at £53.22. Initial psychology sessions were post-trial. Overall, delivering the CCM, including all staff and specialities, cost £3457.78 in 6 months, averaging £44.33 per participant per month.
Discussion
The aim of this study was to assess the feasibility and acceptability of conducting a future definitive RCT to evaluate the clinical and cost-effectiveness of implementing a CCM for integrating physical and mental healthcare in an MSK setting. The trial met the minimum criteria for consent and recruitment rates, as per predefined progression criteria. However, it did not meet the minimum requirements for adherence (58.8% vs target of 75%) or retention, with 37.5% of participants withdrawing by the final 6-month follow-up. Withdrawal rates were slightly higher in the usual care arm (40%) compared with the intervention arm (35%).
A future RCT will first require a pilot study to explore a more robust retention strategy. Maintaining participant retention and adherence to case manager appointments is a commonly cited challenge associated with implementing an RCT design and can be costly.34 These challenges were evident in the current feasibility trial as potential features impacting retention and adherence were multifactorial, although principally attributed to the limited infrastructure and resources available to maintain adherence.34 35 Although the research team employed reminders via phone messages and calls, additional strategies are needed for future trials. Options include non-financial incentives, improved tracking methods, such as clinic and home outreach for challenging-to-locate participants, and covering travel and parking costs.36
Additionally, improving communication with participants between appointments by using a text message service or trial newsletters,37 and considering participant communication preferences from the start will be beneficial. Recruitment occurred in a tertiary NHS hospital, where patients with complex physical needs are referred, which could, potentially lead to higher rates of mental health conditions compared with primary or secondary care settings.38 39 It is possible that recruitment rates would be higher in a tertiary care setting due to their greater healthcare needs, hence caution is needed when generalising the findings to other healthcare settings. A future trial should consider employing two full-time research professionals with flexible hours for equitable recruitment and follow-up, accommodating patients in full-time employment.
Despite challenges with maintaining retention and adherence, healthcare professionals and patients largely embraced the trial. Facilitators included feeling heard by the case manager and the research team, as well as having access to appropriate psychiatric care (intervention participants). However, barriers involved waiting times for psychological appointments and limited formal communication between the case manager and other healthcare professionals, which must be taken in consideration in a future trial. These observations reflect findings from existing systematic reviews that have explored perspectives of physiotherapists towards the integration of physical and mental healthcare40 41 evaluated the knowledge, behaviours, attitudes and beliefs of physiotherapists towards their use of psychological interventions. While physiotherapists hold positive views towards the integration of psychological interventions among their standard practice, barriers to implementation exist, including time constraints and clarity of role. The impact of these barriers might also vary depending on the specific interventions/healthcare models used to facilitate integration.
As the agenda to improve the care of patients with MSK and coexisting mental health conditions continues to grow,42 a wide range of integrative healthcare interventions have been developed and evaluated. These interventions can be broadly categorised according to; in-person multidisciplinary, self-management, digital, education-based and telephone based interventions. However, there is no clear overall consensus regarding their superiority or evidence of routine implementation.42 The CCM has evolved as a standard component of multiple other physical healthcare settings and populations, with robust evidence to demonstrate its effectiveness for achieving clinically meaningful improvements in mental health symptoms that coexist alongside diabetes, cancer, cardiac disease and stroke.43 44 The basis of the model, which includes proactive screening, coordination of care and timely follow-up might all contribute towards the overall effectiveness of the intervention. While it is not possible to quantify the efficacy of individual components, the health-related benefits seen in many other conditions/settings, along with the largely positive experiences observed within the current feasibility trial, warrant further investigation of the CCM within the context of chronic MSK pain.
Proposed changes to intervention
The qualitative findings offer valuable insights to enhance a future trial. Defining the case manager’s role comprehensively,45 specifying communication frequency and establishing formal agreements with protected time are vital to manage expectations and ensure timely intervention. Establishing a formal agreement with protected time is crucial for timely assessment and intervention for psychology department referrals. Funding allocation for a part-time psychologist could improve patient support within the intervention arm, enhancing implementation and communication. Nevertheless, inadequate funding resulted in some participants missing therapy, diluting the intervention’s impact. Due to growing evidence to support the effectiveness of psychological interventions delivered by allied health professionals (AHPs),46 consideration could be made to train AHPs, such as physiotherapists and occupational therapists, to take on the case manager’s role.
Proposed changes to methodology
Several modifications are proposed. To emphasise physical improvement through mental health optimisation, consider MSK-HQ as the primary outcome in future research. It may also be beneficial to add quantitative secondary outcomes of changes in physical health such as grip strength as a global measure of physical strength as well as opioid use preintervention and postintervention.47 Incorporate the Client Service Receipt Inventory (CSRI)48 to calculate service use costs. Include open-ended questions at the final follow-up in patient interviews to boost retention and assess the study’s social value49 by providing person-centred care and ensuring participants feel heard.50 To prevent contamination, explore a cluster randomised design between the intervention and usual care arms.
Conclusions
This feasibility trial offers valuable evidence that clinicians and participants in both arms valued the trial for facilitating integration of physical and psychological care. This trial demonstrates the feasibility of recruiting to the CCM within a tertiary care centre setting. While retention and adherence rates fell short of expectations, robust retention strategies can mitigate this in a future trial. Qualitative data informed modifications to enhance the intervention, delivery model and study design for a future multicentre trial.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and ethical approval was granted by Cambs and Herts Research Ethics Committee 21/EE/0257. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The authors would like to express their sincere gratitude to the participants, patients and professionals, who gave their time to participate in this study. Thanks to Lucy Dove who contributed to the original idea, shaped the protocol and supported the funding application and Dr Catherine Minns Lowe for her valuable feedback on the protocol and support/considerations for a future multicentre RCT.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @mariajoaoresend
Contributors AJ and PR initiated and conceptualised the study. AJ, MJCT, PR and RT designed and constructed the study protocol and made substantial contributions to the conception or design of the work, acquisition, analysis and interpretation of data. MJCT and RA wrote the main manuscript text. MJCT created tables, images and online supplemental material. All authors reviewed the manuscript, tables, images and online supplemental materials. RA and RT proofread all documents. All authors (AJ, MJCT, PR, RA and RT) were involved on the final approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved among all authors. PR is the author guarantor.
Funding This work was supported by Royal National Orthopaedic Hospital Charity (1166129).
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.