Article Text
Abstract
Objectives Investigate the correlation between the percentage of predicted forced expiratory volume in 1 s (FEV1%pred) and survival outcomes, namely relapse-free survival (RFS) and overall survival (OS), in patients diagnosed with operable early-stage non-small cell lung cancer (NSCLC).
Design Prospective observational study.
Setting Clinical settings in Xiangya Hospital, Central South University, Hunan, China.
Participants From November 2014 to December 2019, 775 operable patients with NSCLC, median age 58 years (IQR 13) with 40.6% women, were consecutively enrolled and underwent preoperative FEV1 assessment. All participants were evaluated using the FEV1%pred assessment. Subsequent treatments and operative interventions followed established protocols for NSCLC.
Results During the follow-up, which lasted a median of 40 months (range 1–85 months) and continued until December 2021, 202 patients either relapsed or died. Optimal FEV1%pred cut-off was identified using receiver operating characteristic analysis. Results revealed 110 and 71 relapses and deaths per 1000 person-years for patients with FEV1 values of <82%pred and ≥82%pred, respectively. Cox proportional hazards models, adjusted for demographics, medical history and surgery characteristics with propensity score overlap weighting, revealed the significant impact of lower FEV1%pred on decreased RFS and OS. An FEV1%pred less than 82% displayed a significant association with decreased RFS (weighted HR, 1.55; 95% CI, 1.14 to 2.09; p=0.007) and OS (weighted HR, 1.50; 95% CI, 1.01 to 2.23; p=0.04).
Conclusions Lower FEV1%pred values notably correlate with compromised RFS and OS in individuals operable for early-stage NSCLC, suggesting that FEV1%pred may serve as a valuable tool in evaluating and managing long-term recurrence risk in patients with early-stage NSCLC.
Trial registration number ChiCTR2100048120.
- Lung Diseases
- Respiratory tract tumours
- Respiratory Function Test
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study adopted the propensity score overlap weighting technique to mitigate the impact of extreme propensities on model outcomes, which is a standardised and published methodology.
This study exclusively enrolled patients who underwent preoperative cardiopulmonary exercise testing, which could introduce selection bias.
Background
In 2020, lung cancer was the leading cause of cancer-related deaths worldwide, resulting in approximately 1.8 million fatalities and 2.2 million new diagnoses. The vast majority of these cases, ranging from 80% to 85%, are categorised as non-small cell lung cancer (NSCLC).1 For individuals diagnosed with early-stage NSCLC, operative lung resection is widely regarded as the most effective oncological treatment option. However, patients with lung cancer remain at a significant risk of relapse and death within a 5-year span post-diagnosis.2 Up to 32–66% of patients with NSCLC relapse or die after surgery,3 and about 83% of relapses are metastatic, which significantly affects the long-term survival of patients. Identifying those patients with NSCLC who are at a heightened risk of disease recurrence or mortality might help with clinical decision-making and personalised intervention strategies.
British Thoracic Society recommends that suitability for surgery should be assessed based on age, cardiovascular health, nutritional and exercise status and respiratory function.4 The forced expiratory volume in 1 s (FEV1) stands as a critical clinical physiological parameter, indicating the volume of air exhaled during the initial second of a forced exhalation following maximal effort.5 FEV1 is one of the most useful parameters in pulmonary function tests for the assessment of patients with lung resection, which is convenient and non-invasive.6 The percentage of predicted FEV1 (FEV1%pred) is derived by dividing the actual FEV1 value by its predicted norm, which is adjusted for age, sex and height.7 Recent research increasingly acknowledges FEV1%pred as not only a marker of impaired lung function but also as a potential predictor of perioperative morbidity, particularly in patients with lung cancer.8
Despite its emerging significance, there is a scarcity of studies examining the relationship between FEV1%pred and overall survival (OS) in the context of post-lung resection patients, with only four such studies reported to date, yielding inconsistent findings.9–12 Three of these studies have identified a correlation between FEV1%pred and OS in patients with lung cancer, although with varying threshold values,10–12 while one study found no significant link between FEV1%pred and postoperative mortality.9 Furthermore, to the best of our knowledge, the association between FEV1%pred and postoperative relapse in patients with NSCLC remains unexplored. Considering the high availability and non-invasive procedure of FEV1%pred detection, investigating the role of FEV1%pred in predicting relapse-free survival (RFS) and OS holds promise and is of clinical significance.
In this prospective observational study, we carried out a relatively large sample size to explore the correlation between FEV1%pred and long-term RFS as well as OS in individuals diagnosed with operable early-stage NSCLC, by adopting propensity score overlap weighting technique to mitigate the impact of extreme propensities.
Materials and methods
Study design and participants
This prospective observational research was conducted as a component of the Xiangya Hospital Exercise Testing (X-ET) project.13 Individuals with suspected NSCLC who were slated for lung resection and underwent the preoperative FEV1 evaluation were consecutively included in the study between 1 November 2014 and 31 December 2019. In the screening process, individuals with small cell lung cancer, benign tumours, advanced-stage (IIIB and IV) NSCLC, chronic obstructive pulmonary disease (COPD), emphysema and those with a prior history of other malignancies were excluded. Additionally, the study has been registered in the Chinese Clinical Trial Registry with the unique identification number ChiCTR2100048120, and we have adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting our work.
Preoperative FEV1 assessment
The preoperative FEV1 values were measured during preoperative cardiopulmonary exercise testing using the CARDIOVIT system (Schiller Switzerland). To ensure the equipment quality, daily volume and flow verification were conducted. We established the acceptability and usability criteria for FEV1 and forced vital capacity (FVC) based on the guidelines provided by the American Thoracic Society and the European Respiratory Society.14
Definition of exposed group and outcomes
We determined the ideal threshold for FEV1%pred as the primary outcome through receiver operating characteristic (ROC) curve analysis and the calculation of the Youden index.15 Individuals with FEV1%pred values below the designated threshold were categorised into the exposed group. FEV1%pred = (FEV1 observed/FEV1 predicted) × 100. Predicted FEV1 was calculated by the following equations16: FEV1 predicted = 4.301 × height (m) − 0.029 × age (years) − 2.492, male; FEV1 predicted = 3.953 × height (m) − 0.025 × age (years) − 2.604, female. FEV1%pred = (FEV1 observed/FEV1 predicted) × 100.
The primary outcome was RFS. Follow-up time referred to the period from the day of lung resection to the incidence of either relapse or death or to 31 December 2021. The secondary outcome was OS. The time-to-death event extended from enrolment to death from any cause. Verification of relapses and deaths involved a three-pronged approach, which included (1) gathering data from the Residence Registration Office, (2) examining the electronic medical record system and (3) conducting follow-up by contacting the families of the study participants.
Covariates
Age, gender, body mass index and smoking history information were gathered on enrolment. Data concerning tumour histology, clinical stage, type of lung resection and factors that might be associated with RFS and OS were obtained from the electronic medical record system following the surgical procedure.
Sample size
In this study, given the absence of an exact comparable study, we referenced a similar study to estimate the effect size.11 Based on an estimated HR of 1.5, a desired power of 0.8, and the occurrence of 202 events in our cohort, we used the Cox proportional hazards model to calculate the required sample size. The total estimated sample size needed for the study is approximately 734 participants, with this number encompassing both the control and exposure groups.
To guarantee the accuracy of the proposed model, it is advisable to have at least 10 equivalent events for each adjusted covariate.17 The presence of 202 observed relapses or deaths in the present study was sufficient for the development of our regression models, allowing for the adjustment of up to 20 covariates. Moreover, we employed the propensity score overlap weighting technique for covariate adjustment, which combines and assigns weights to all covariates as a single regression variable.
Statistical analysis
The Shapiro-Wilk test was conducted to assess the normality of continuous variables. Non-normally distributed continuous variables were presented as the median and IQR, while normally distributed continuous variables were described as mean±SD. Categorical variables were reported as counts (percentages). Standardised mean difference (SMD) was used to gauge the balance of individual covariates both before and after applying the propensity score overlap weighting technique.18 Conventionally, an SMD less than 0.1 is considered adequate for balance.18 Additionally, a Love plot was created to display covariate balance before and after the weighting.
The propensity score model with covariates was established using the overlap weighting method to mitigate the impact of extreme propensities on model outcomes.19 Weight assignment in overlap weighting was done based on the probability of individuals belonging to the alternative group.19
Kaplan-Meier curves were generated for both RFS and OS. To evaluate the statistical significance of the differences between the low and high FEV1%pred groups in the survival curves, the log-rank test was employed. Cox proportional hazards models were used to examine the relationship between FEV1%pred and RFS as well as OS, both before and after adjusting for covariates through propensity score overlap weighting. Furthermore, we calculated both the crude and weighted incidence rates, representing the relapse or mortality rate per 1000 person-years.
The association between FEV1%pred and RFS and OS was internally validated using bootstrapping. Briefly, 1000 bootstrap samples were generated, and the Brier scores, calibration slope and C-index were estimated to assess the Cox proportional hazards models’ overall performance, agreement and efficiency in distinguishing patients at high and low risks of relapse and death.20
An examination of interactions was conducted for all covariates (recorded confounding factors), and the E-factor was used to evaluate unmeasured confounders.21 Furthermore, predefined subgroup examinations were executed considering birth gender, age, body mass index (BMI and tobacco use status. Propensity scores with overlap weighting were regenerated prior to creating the subgroup model. Considering the risk of type I errors arising from multiple comparisons, the subgroup analyses were regarded as exploratory.
The analysis was conducted using R software (V.4.2.0). We made use of packages such as pROC, PSweight, tableone, cobalt, survival, survminer, fmsb, rms, boot and E-value. Statistical significance was set at p<0.05 (two-sided). The code and relevant references for the statistical analyses are openly accessible on the GitHub repository at this URL: https://github.com/YSDun/FEV1/blob/101e5cd567302c76d0c79e00cfa4c953667bbd10/FEV1.Rmd
Results
Characteristics of the participants
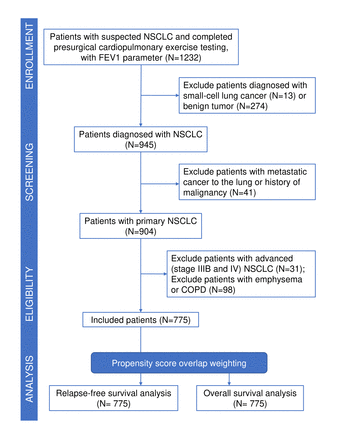
Between 1 November 2014 and 31 December 2019, a cohort of 1232 patients with suspected NSCLC underwent preoperative cardiopulmonary exercise testing, with an assessment of their FEV1 values. Following the initial screening process, 775 patients met the eligibility criteria and were enrolled in the study. Meanwhile, 457 individuals were excluded from the study cohort due to benign tumours (n=274), small cell lung cancer (n=13), lung metastases originating from sites other than the lung (n=41), NSCLC at stages IIIB and IV (n=31) and emphysema or COPD (n=98) (figure 1). The median age of the 775 participants was 58 years (IQR, 13 years), with a predominant male representation (59.4%). The online supplemental table 1 displays the characteristics of FEV1, FVC and maximal ventilatory volume. ROC analysis, along with the Youden index, determined an FEV1 value of <82% of the predicted value as the optimal prognostic cut-off in this investigation, as depicted by the ROC curves in online supplemental figure 1. Out of the 775 participants, 234 (30.2%) exhibited an FEV1 below 82% of the predicted value, while 541 (69.8%) demonstrated an FEV1 equal to or greater than 82% of the predicted value. The mean FEV1%pred was 89 (SD, 16).
Supplemental material
Study review from enrolment to analysis. COPD, Chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; NSCLC, non-small cell lung cancer.
Male participants, older age, squamous cell lung cancer diagnosis, advanced clinical stages, lower MET levels and a history of smoking were associated with lower FEV1% predicted values. For more details, refer to table 1. Online supplemental figure 1 displays covariate balance before and after propensity score overlap weighting.
Characteristics of operable patients with early-stage NSCLC, before and after applying propensity score overlap weighting
The association between FEV1%pred and RFS
Over the study period, 202 patients faced relapse or death. The relapse and death rates were 110 and 71 per 1000 person-years for patients with FEV1 <82%pred and ≥82%pred, respectively (weighted incidence rate difference (95% CI), 39 (25 to 78) events per 1000 person-years). Patients with FEV1 <82%pred had a 1.55-fold higher risk of relapse or death than those with FEV1 ≥82%pred (weighted HR (95% CI), 1.55 (1.14 to 2.09)) (table 2). The Kaplan-Meier curve for RFS is presented in figure 2a.
Kaplan-Meier estimates of the patients with NSCLC with FEV1 <82%pred and FEV1 ≥82%pred (N=775). Kaplan-Meier curves for relapse-free survival (RFS) (a); Kaplan-Meier curves for overall survival (OS) (b). FEV1, forced expiratory volume in 1 s; NSCLC, non-small cell lung cancer. The shadow, along with the curves, represents the 95% CI.
Association of FEV1%pred with RFS and OS in operable patients with early-stage NSCLC
Several sensitivity analyses were conducted to assess the potential sources of bias that might affect the observed association between FEV1%pred and RFS. First, interaction tests were conducted to assess the influence of the measured covariates. Among these covariates, only BMI showed a significant interaction (p=0.03), while the remaining covariates did not exhibit significant interactions (all p>0.05) (online supplemental table 2). Prespecified subgroup analyses consistently showed that the association between FEV1%pred and RFS remained stable across the subgroups of older adults, males, individuals with high BMI and those with a history of smoking (figure 3a). The E-value was computed to assess potential interference from unknown confounding factors. The resulting E-value was 2.05, suggesting that residual confounding could explain the observed relationship between FEV1%pred and RFS if there exists an unmeasured covariate with a relative risk association of at least 2.05 with both impaired FEV1 and relapse or death. However, most other risk factors for relapse or death in patients with NSCLC have an HR of 1.10 or lower,22 which is considerably smaller than 2.05. This suggests that the observed association between FEV1%pred and RFS is less likely to be attributable to an unmeasured confounder.
Relapse-free survival (a) and overall survival (b) in patient subgroups with FEV1 <82%pred vs FEV1 ≥82%pred. BMI, body mass index; FEV1, forced expiratory volume in 1 s.
We performed internal validation through bootstrapping resampling. Our analysis yielded a Brier score of 0.18 (95% CI, 0.17 to 0.19), a calibration slope of 0.79 (95% CI, 0.66 to 0.92) and a C-index of 0.57 (95% CI, 0.52 to 0.62), indicating strong overall performance of the FEV1%pred and RFS regression model (online supplemental table 3).20
The association between FEV1%pred and OS
Over the study duration, 112 participants experienced mortality, with mortality rates of 57 and 38 deaths per 1000 person-years among patients with low and high FEV1%pred, respectively. The weighted incidence rate difference in terms of death between the two groups was 19 (95% CI, 2 to 41) deaths per 1000 person-years. Those with FEV1 <82% predicted had a 1.50 times higher mortality risk than those with FEV1 ≥82% predicted (weighted HR (95% CI), 1.50 (1.01 to 2.23)). The Kaplan-Meier curve of OS is presented in figure 2b.
No significant interactions between covariates and the association of FEV1%pred with OS were observed (all p>0.05), except for BMI (p=0.01) (online supplemental table 2). The relationship between FEV1 and OS remained consistent in male and high BMI subgroups (figure 3b). The E-value was 1.98, whereas most other risk factors had an HR of 1.2123 or lower for death, which is considerably smaller than 1.98. This finding suggests that the observed association is unlikely to be attributable to an unmeasured confounder.
Internal validation using bootstrapping confirmed the association between FEV1%pred and OS. The Brier score was 0.12 (95% CI, 0.11 to 0.13), the calibration slope was 0.71 (95% CI, 0.65 to 0.77) and the C-index was 0.55 (95% CI, 0.52 to 0.58), signifying the favourable overall performance of the FEV1%pred and OS regression model (online supplemental table 3).20
Discussion
This study employs the largest sample size to date for examining the relationship between FEV1%pred and OS in individuals with stage I to IIIA NSCLC who have undergone lung resection. Our results demonstrate that decreased FEV1%pred is significantly linked to shorter RFS and OS in operable patients with early-stage NSCLC (online supplemental file 2). These findings suggest that FEV1%pred could be a valuable instrument for evaluating long-term prognosis of individuals with early-stage NSCLC.
Supplemental material
There are several debates surrounding the utilisation of FEV1%pred in forecasting the long-term OS in individuals with NSCLC. A notable study by Shen et al 24 involving patients with advanced-stage NSCLC indicated a strong link between diminished FEV1 and reduced OS. Yet, in the context of early-stage NSCLC, the findings are less consistent. Some investigations, such as those conducted on Norwegian (sample size (N)=148) and Danish patients (N=252), have suggested that FEV1%pred serves as a valuable prognostic indicator for predicting long-term prognosis.10 11 Conversely, a study of American patients with stage I NSCLC reported conflicting results.9 This study corroborates the idea that FEV1%pred is a valuable method for evaluating the long-term OS in operable patients with early-stage NSCLC.
The utilisation of FEV1%pred as a prognostic marker for patients with NSCLC is further complicated by the absence of a universally accepted threshold value. Normal FEV1%pred values fluctuate based on multiple factors, including age, height, gender, ethnicity and individual health status.25 Conventionally, an FEV1%pred value below 80% has been regarded as reflecting impaired lung function in the general population.26 Furthermore, findings from recent studies have indicated that the cut-off values of FEV1%pred for predicting mortality risk in individuals with various health conditions exhibit considerable variability.27 A study by Dittrich et al 28 recommended continuous updating of the disease- and purpose-specific FEV1 reference values. In the present study, which involved Chinese patients with early-stage NSCLC who underwent lung resection, with a median age of 59 (IQR, 13), we observed that an FEV1 of <82% predicted was associated with a higher all-cause mortality rate. Further research is required to establish the optimal FEV1%pred cut-off value for predicting long-term outcomes in patients with NSCLC of diverse racial, ethnic and age groups.
Recently, there have been considerable advances in studies that have expanded the use of FEV1 for predicting the incidence and recurrence of lung cancer. For instance, a study reported that impaired FEV1 was associated with lung cancer incidence in the general population.29 Likewise, a retrospective analysis published in 2022 identified an association between decreased FEV1 and an elevated risk of worse RFS in a cohort of 151 patients with advanced NSCLC who were treated with immune checkpoint inhibitors.24 Nonetheless, it is important to highlight that a study published in 2014 did not observe any significant correlation between FEV1 and locoregional RFS or distant metastasis-free survival in patients with NSCLC who received postoperative radiotherapy, despite the fact that low FEV1 was notably linked to a poorer 5-year OS.30 The current study supports that FEV1%pred may serve as a valuable instrument for aiding in the prediction of long-term recurrence risk in patients with early-stage NSCLC.
The underlying mechanisms linking FEV1 and the long-term prognosis of patients with NSCLC remain not fully elucidated. Previous studies have suggested that diminished lung function may lead to systemic hypoxia, potentially affecting the efficacy of cancer treatment, such as reduced chemotherapy tolerance.31 Patients with impaired FEV1 may have different types of abnormal lung function, which may influence the decision to surgically remove the lung lobes, as well as the deterioration of function after surgery.9 It is also plausible that FEV1 decline may reflect tumour biological behaviours. FEV1 levels were linked to the occurrence of lung cancer in a population-based cohort investigation.32 Furthermore, impaired lung function might influence immune function, leading to a compromised anticancer response.33 However, it should be noted that these hypotheses are currently speculative and require further investigation to determine the precise mechanisms involved.
Limitations
This study exhibits several limitations. First, it exclusively enrolled patients who underwent preoperative cardiopulmonary exercise testing, which could introduce selection bias. Second, despite employing the propensity score overlap weighting method and E-value analysis to minimise confounding bias, residual and unmeasured confounding factors may remain. Third, data on neoadjuvant radiotherapy or chemoradiotherapy were not obtained, and data on specific treatment for relapsing patients or the mutation status of the tumours were unavailable, but adjustments were made for factors such as TNM (tumour, node, metastases) classification, accessibility and eligibility for neoadjuvant therapy. Fourth, as RFS and OS are determined by multifaceted conditions, the use of FEV1%pred in a comprehensive preoperative algorithm is necessary to accurately evaluate the risk of relapse and death. Additional research is warranted to ascertain and assess the prognostic significance of combining FEV1%pred with other predictive factors in patients with NSCLC.
Conclusion
Reduced preoperative FEV1%pred was significantly associated with worse RFS and OS among operable patients with early-stage NSCLC. The findings suggest that FEV1%pred could be a useful tool for aiding in the prediction and management of long-term recurrence risk in patients with early-stage NSCLC.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by Ethics Committee of Xiangya Hospital, Central South University, reference number 202010145. Given that this research did not impact patient management, the requirement for informed consent was waived by the Ethics Committee.
References
Footnotes
X @CambsRespPhys
SW and SF contributed equally.
Contributors SW and SF conceptualised and designed the study. SW, JWR-G, NC, YDu, KS, NX, SF, BY, YDun and SL collected data. SW, JWR-G, NC and YDu analysed the data. SW and JWR-G were responsible for the initial draft of the manuscript. All authors provided comments on earlier drafts of the manuscript, and they all reviewed and approved the final version. YDun, JC and SL are guarantors. SW and SF contributed equally to the work.
Funding This study was supported by the National Natural Science Foundation of China (82172549 to SL, 82272613 to YDun and 82101407 to YDu) and Shenzhen Yantian District Science and Technology Project (YTWS20210101 to JC).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.