Article Text
Abstract
Introduction Neoadjuvant chemotherapy has been demonstrated to be effective and recommended as the standard treatment option in patients with locally advanced gastric or gastro-oesophageal junction (G/GOJ) cancer. In this study, we will explore the efficacy and safety of chemotherapy combined with cadonilimab, a programmed death-1/cytotoxic T lymphocyte-associated antigen-4 bispecific antibody, in neoadjuvant therapy for locally advanced G/GOJ adenocarcinoma.
Methods and analysis This is a single-centre, single-arm, open-label, phase II trial that will enrol 37 patients in total. Eligible patients will be registered and receive three cycles of oxaliplatin and S-1 (SOX) regimen in combination with cadonilimab. Radical D2 (D2 lymphadenectomy) gastrectomy will be performed within 4 weeks after the last administration of chemotherapy plus cadonilimab. The primary endpoint is the pathological complete response rate. Secondary endpoints are R0 resection rate, major pathological response, 2-year disease-free survival rate, 2-year overall survival rate and safety. The first participant was recruited on 1 September 2023 and the enrolment will be completed in July 2025.
Ethics and dissemination Written informed consent will be required from and provided by all the patients enrolled. The study protocol (V.3.0, 28 April 2023) has been approved by the independent ethics committee of West China Hospital, Sichuan University (approval number: 2023526) and conducted under the Declaration of Helsinki. The results of the study may provide more evidence for neoadjuvant immunotherapy combined with chemotherapy in locally advanced G/GOJ adenocarcinoma.
Trial registration number ClinicalTrials.gov, NCT05948449.
- Gastrointestinal tumours
- CHEMOTHERAPY
- Clinical Trial
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Inclusion/exclusion criteria are compatible with the study objectives.
Simon’s two-stage Minimax approach will minimise the sample size.
Simon’s two-stage optimal approach will allow for early termination due to the absence of treatment efficacy in the first stage.
The study is a single-arm, open-label, phase II clinical trial with no control group.
Introduction
Gastric or gastro-oesophageal junction (G/GOJ) cancer is the fifth most commonly diagnosed cancer and the fourth leading cause of cancer-related deaths. In 2020, over one million new cases of G/GOJ cancer and 769 000 deaths due to G/GOJ cancer were estimated to occur globally.1 Surgery is still the main curative treatment for G/GOJ cancer; however, most of patients undergoing gastrectomy will experience disease recurrence due to residual tumour or tumour micrometastasis.2
Neoadjuvant therapy may significantly reduce the tumour burden, increase the R0 resection rate, reduce the postoperative recurrence rate and evaluate the tumour response to the treatment regimen. Currently, neoadjuvant therapy has been widely applied to the treatment of a series of cancers, such as breast, rectal, oesophageal, head and neck, lung, prostate, and many other cancer types.3–6 The MAGIC Study from the UK established the role of neoadjuvant chemotherapy in gastric cancer, while the PRODIGY Study in South Korea, the RESONANCE Study and the RESOLVE Study in China further confirmed that neoadjuvant chemotherapy can improve the R0 resection rate and prognosis of patients with gastric cancer without increasing the incidence of severe postoperative complications.7–10 Although studies have demonstrated the clinical benefit of neoadjuvant chemotherapy for G/GOJ cancer, the pathological complete response (pCR) and long-term survival rates are still unsatisfactory.
Immune checkpoint inhibitors (ICIs), which target programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1) and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), have shown promising antitumor effects and revolutionised the treatment of malignancies. Recently, the combination of ICIs and chemotherapy has shown prolonged overall survival (OS) and progression-free survival (PFS) compared with chemotherapy alone and reduced the risk of death by 20%–35% in the first-line setting of patients with advanced or metastatic G/GOJ cancer.11 12 The inspiring results of these studies give us rational reasons to further investigate the application of this strategy in neoadjuvant therapy of patients with locally advanced G/GOJ cancer. In a single arm, phase II clinical study, Jiang et al evaluated the efficacy of sintilimab combined with capecitabine and oxaliplatin (XELOX) regimen in locally advanced, resectable G/GOJ adenocarcinoma. A total of 36 patients were enrolled, all of whom underwent surgery, with an R0 resection rate of 97.2%. The pCR and major pathological response (MPR) rates were 19.4% (95% CI 8.8% to 35.7%; 90% CI 10.7% to 33.1%) and 47.2% (95% CI 31.6% to 64.3%), respectively. The disease-free survival (DFS) and OS at 1 year were 90.3% (95% CI 80.4% to 100.0%) and 94.1% (95% CI 86.5% to 100.0%), respectively.13 In another similar study, the combination of tislelizumab and oxaliplatin and S-1 (SOX) also showed promising efficacy and safety in neoadjuvant treatment for locally advanced, resectable G/GOJ adenocarcinoma. Thirty-two patients were included in the study. Seventeen patients (53.1%) obtained MPR (≤10% survival tumour cells) and eight patients (25%) achieved pCR. The annual relapse-free survival (RFS) and OS were 90.0% and 91.4%, respectively.14 These results provide new insight and suggest that chemotherapy combined with immunotherapy may represent a promising therapy for the neoadjuvant treatment of locally advanced, resectable G/GOJ adenocarcinoma.
Cadonilimab (AK104), a novel tetrameric form of PD-1/CTLA-4 bispecific antibody, could retain the efficacy benefit derived from the combination of anti-PD-1 and anti-CTLA-4 while conferring superior safety compared with the coadministration of these individual agents. In recent studies, cadonilimab has shown excellent efficacy and safety in various tumours, including cervical, nasopharyngeal, liver and other cancers. On 29 June 2022, cadonilimab was approved in China for patients with recurrent or metastatic cervical cancer who failed previous platinum-based chemotherapy.15 In addition, the efficacy and safety of cadonilimab combined with chemotherapy as first-line treatment for patients with advanced or metastatic G/GOJ adenocarcinoma was announced at the 2022 American Society of Clinical Oncology Gastrointestinal Oncology Symposium. A total of 96 patients were included, and 88 patients (92%) underwent at least one tumour assessment. The objective response rate was 65.9% (58/88), with 2 cases (2.3%) of complete response and 56 cases (63.6%) of partial response. The disease control rate was 92.0% (81/88). The median PFS was 7.10 months (95% CI 5.55 to 10.48) and the median OS was 17.41 months (95% CI 12.35 to not estimable (NE)).16 The above results confirm that the combination of cadonilimab and chemotherapy is a potential new treatment option in the first-line setting of gastric cancer.
Inspired by these findings, we conduct a single-arm, phase II trial to explore the efficacy and safety of chemotherapy combined with cadonilimab in neoadjuvant therapy for locally advanced G/GOJ adenocarcinoma. Moreover, we will also explore the predictive biomarkers of immunotherapy response, and establish a predictive model to screen out patients who might benefit from the neoadjuvant immunotherapy-chemotherapy regimen.
Methods and analysis
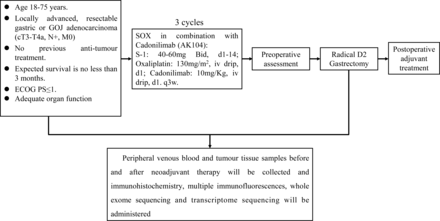
This is a single-centre, single-arm, open-label, phase II trial designed to assess the efficacy and safety of chemotherapy combined with cadonilimab in neoadjuvant therapy for locally advanced G/GOJ adenocarcinoma. Participants will be enrolled from West China Hospital, Sichuan University, Chengdu, China. All participants are required to provide written informed consent before enrolment (online supplemental material). The study has been prospectively registered at ClinicalTrials.gov (NCT05948449). The first participant was recruited on 1 September 2023, and enrolment should be completed in July 2025. Figure 1 shows an overview of the study design.
Supplemental material
Study flow chart. D2, D2 lymphadenectomy; ECOG, Eastern Cooperative Oncology Group; GOJ, gastro-oesophageal junction; PS, performance status; SOX, oxaliplatin and S-1.
Endpoints
The primary endpoint is the pCR rate. Secondary endpoints are R0 resection rate, MPR, 2-year DFS rate, 2-year OS rate and safety profile. Exploratory endpoints are predictive biomarkers of immunotherapy response. The primary analyses will be performed in the intention-to-treat (ITT) population. All adverse events (AEs) will be analysed in the safety population, defined as patients administered at least one dose of neoadjuvant treatment. Neoadjuvant or adjuvant treatment emergent AEs will be reported separately due to the different regimens applied. Surgery-related morbidity and mortality will be analysed in the per-protocol population, defined as patients who were compliant with the study protocol and proceeded to surgery. To determine whether high pCR rates can translate into longer survival, follow-up of these participants will last for at least 2 years.
Study population and eligibility criteria
Inclusion criteria:
Age 18–75 years.
Histologically or cytologically confirmed diagnosis of locally advanced G/GOJ adenocarcinoma (cT3-T4a, N+, M0) as assessed by exploratory laparoscopic surgery, ultrasonography and/or CT/MRI.
Resectable G/GOJ cancer, as judged by experienced surgeons.
There has been no previous antitumour treatment.
The expected survival is more than 3 months.
Eastern Cooperative Oncology Group (ECOG) performance status (PS)≤1.
Adequate organ function including the following:
Total bilirubin ≤1.5 times the upper limit of normal (ULN).
Aspartate transaminase and alanine transaminase ≤3 × ULN.
Alkaline phosphatase ≤2.5 × ULN (if the tumour invaded the liver, ≤3 × ULN).
Serum creatinine ≤1.5 × ULN.
Serum amylase and lipase ≤1.5 × ULN.
International standardised ratio/partial thromboplastin time ≤1.5 × ULN.
Platelet count ≥75 000/mm3.
Haemoglobin ≥9 g/dL.
Absolute neutrophil count ≥1500/mm3.
Strict contraception.
Patients must be able to understand and be willing to sign the written informed consent form. A signed informed consent form must be appropriately obtained prior to the conduct of any trial-specific procedure.
Exclusion criteria:
Unable to comply with the research programme or procedures.
Undergoing other drug clinical trials or having participated in any drug clinical trials 1 month before enrolment.
Active autoimmune disease or history of refractory autoimmune disease.
Receiving corticosteroids (>10 mg/day prednisone or equivalent dose of steroids) or other systematic immunosuppression therapies within 14 days before enrolment, excluding the following therapies: steroid hormone replacement therapy (≤10 mg/day); local steroid therapy; and short-term, prophylactic steroid therapy for preventing allergies or nausea and vomiting.
Active or clinically significant cardiac disease:
Congestive heart failure >New York Heart Association class II.
Active coronary artery disease.
Arrhythmias requiring treatment other than β-blockers or digoxin.
Unstable angina (with angina symptoms at rest), new angina within 3 months before enrolment or new myocardial infarction within 6 months before enrolment.
Evidence or history of bleeding diathesis or coagulopathy.
Grade 3 bleeding events 4 weeks before enrolment.
Thromboembolism or arteriovenous events, such as cerebrovascular events (including transient ischaemic attack), deep vein thrombosis or pulmonary embolism, occurred 6 months before enrolment.
Currently taking anticoagulants.
Other tumours that have not been treated or exist at the same time, except carcinoma in situ of the cervix, treated basal cell carcinoma or superficial bladder tumour. If the tumour was cured and no evidence of disease was found for more than 3 years, the patient can be enrolled. All other tumours must be treated at least 3 years before enrolment.
Patients with pheochromocytoma.
Patients with a history of HIV infection or active hepatitis B/C.
Ongoing > level 2 infection.
Symptomatic brain metastasis or meningioma.
Unhealed wounds, ulcers or fractures.
Patients with renal failure requiring blood or peritoneal dialysis.
Dehydration ≥ 1 grade.
Epileptic that needs medication.
Active, symptomatic interstitial pneumonia, pleural or ascites that causes dyspnoea (dyspnoea ≥ grade 2).
History of organ transplantation (including corneal transplantation).
Allergic to research drugs or similar drugs, or suspected allergies.
Patients with malabsorption.
Pregnant or lactating women.
Those patients that the investigator believes are not suitable for the study.
Medical, psychological or social conditions can affect the recruitment of patients and evaluation of study results.
Other antitumour therapy (chemotherapy, radiotherapy, surgery, immunotherapy, biotherapy, chemoembolisation) other than investigator drugs. Palliative external irradiation for non-target lesions is allowed.
Previously used oxaliplatin, S-1 or cadonilimab.
Major surgery 4 weeks before recruitment, open biopsy or major trauma surgery (excluding biliary stents or percutaneous biliary drainage).
Treatment with antitumour Chinese herbal medicine.
History of allogeneic blood transfusion within 6 months.
Intervention
Laparoscopic exploration should be performed to detect occult peritoneal metastases and inspect the primary lesion, liver, diaphragm, pelvic organs, bowel and omentum. Patients who meet the inclusion criteria will be enrolled and sign the informed consent form. Then, three cycles of neoadjuvant therapy will be administered: S-1: 40–60 mg two times per day, d1–14, once every 3 weeks; oxaliplatin: 130 mg/m2, intravenous drip, d1, once every 3 weeks; cadonilimab (AK104): 10 mg/kg, intravenous drip, d1, once every 3 weeks. Antiemetic, acid suppressing and anti-allergic treatments are allowed. Radical D2 (D2 lymphadenectomy) gastric cancer resection will be performed within 4 weeks after the last administration of chemotherapy plus cadonilimab. Afterwards, we will recommend the best postoperative adjuvant treatment, and the adjuvant regimen will be determined by the patients themselves. Follow-up will be conducted every 3 months (according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 standards) until tumour recurrence, death or 2 years after surgery.
Statistical analysis
The sample size was calculated based on the assumption that the pCR rate of neoadjuvant SOX chemotherapy is 5.6%.10 A total of 33 patients treated with neoadjuvant cadonilimab in combination with SOX regimen will provide 80% power to detect a pCR rate of 20% at a one-sided 5% alpha level. Considering a 10% discontinuation rate, 37 assessable patients will be enrolled in the study. Descriptive statistics of baseline and clinicopathological characteristics will be performed. The pCR, MPR and R0 resection rate will be calculated, and the corresponding CIs will be estimated by the Blaker’s binomial exact method. Kaplan-Meier estimates of DFS and OS probabilities will also be determined, with respective 95% CIs. All efficacy analyses will be performed in the ITT population and all AEs will be analysed in the safety population, defined as patients administered at least one dose of neoadjuvant treatment.
Patient and public involvement
None.
Ethics and dissemination
Written informed consent will be required for patients enrolled. This study will be conducted under the Declaration of Helsinki, without causing any extra harm or risks to patients. The protocol (V.3.0, 28 April 2023) has been approved by the independent ethics committee of West China Hospital, Sichuan University (approval number: 2023526) and has been prospectively registered on ClinicalTrials.gov with registration number NCT05948449. The results of the study will be presented at international oncology congresses and published in peer-reviewed journals.
Discussion
G/GOJ cancer remains one of the most commonly diagnosed malignancies worldwide. Gastrectomy is the main curative treatment for G/GOJ cancer; however, disease recurrence or metastasis will occur in most patients undergoing gastrectomy. In recent decades, a number of studies have investigated the efficacy of adjuvant chemotherapy in patients with resectable gastric cancer. In the ACTS-GC Trial, 1059 patients with stage II or stage III gastric cancer were enrolled and randomly assigned to either the surgery plus S-1 group or the surgery-only group. The 5-year OS rates in the surgery plus S-1 group and surgery-only group were 71.7% and 61.1%, respectively, and the 5-year RFS rates were 65.4% and 53.1%, respectively.17 In another study conducted in South Korea, mainland China and Taiwan (the CLASSIC Trial), 1035 patients with stage II-IIIB gastric cancer who underwent D2 radical gastrectomy were randomly assigned to the adjuvant chemotherapy (XELOX) group or observation alone group. The 5-year DFS rates were 68% and 53%, respectively.18 Based on the results of these two studies, D2 radical gastrectomy with adjuvant chemotherapy has been recommended as the standard treatment option for patients with G/GOJ cancer in stage II–III. However, adjuvant therapy for gastric cancer has entered a bottleneck stage in recent years. Many patients experience short-term recurrence after surgery, especially in patients with stage III gastric cancer.
Neoadjuvant therapy has been widely applied to the treatment of several cancers. The MAGIC Study, PRODIGY Study, and the RESONANCE and RESOLVE Studies also established the role of neoadjuvant chemotherapy in G/GOJ cancer, and neoadjuvant chemotherapy has been recommended in patients with locally advanced, resectable G/GOJ cancer by the European Society for Medical Oncology, National Comprehensive Cancer Network, and Chinese Society of Clinical Oncology clinical practice guidelines. Recently, a number of studies have also evaluated the efficacy of chemotherapy plus PD-1 inhibitors in neoadjuvant therapy of patients with locally advanced G/GOJ cancer. The addition of PD-1 inhibitors significantly increases the pCR rate, R0 resection rate and long-term survival, indicating that chemotherapy plus PD-1 inhibitors is a promising treatment option for the neoadjuvant treatment of locally advanced, resectable G/GOJ adenocarcinoma.13 14
In addition to PD-1 and PD-L1, CTLA-4 is regarded as another key immune checkpoint. CTLA-4 is not expressed on the surface of initial T cells. After T cell activation, CTLA-4 expressed on the surface of activated T cells can competitively bind to ligand B7 with CD28, thereby reducing T cell activation levels and inhibiting T cell proliferation.19 In theory, CTLA-4 monoclonal antibodies and PD-1 monoclonal antibodies can cooperate and coordinate with each other, effectively weakening tumour immune escape and promoting the killing effect of the immune system. Therefore, the combination of CTLA-4 monoclonal antibody and PD-1 monoclonal antibody may provide a strong foundation for dual immune combination therapy in clinical practice. In the CheckMate 067 Study, Hodi et al investigated the efficacy and safety of nivolumab plus ipilimumab or nivolumab alone compared with ipilimumab alone in patients with advanced melanoma.20 Patients with previously untreated, unresectable, stage III or stage IV melanoma were randomly assigned 1:1:1 to the nivolumab plus ipilimumab group, the nivolumab group, or the ipilimumab group. At a minimum follow-up of 48 months from the date that the final patient was enrolled and randomised, the median OS was not reached (95% CI 38·2 to not reached) in the nivolumab plus ipilimumab group, 36·9 months (28·3 to not reached) in the nivolumab group, and 19·9 months (95% CI 16·9 to 24·6) in the ipilimumab group. The median PFS was 11·5 months (95% CI 8·7 to 19·3) in the nivolumab plus ipilimumab group, 6·9 months (95% CI 5·1 to 10·2) in the nivolumab group, and 2·9 months (95% CI 2·8 to 3·2) in the ipilimumab group. These results suggested that nivolumab plus ipilimumab can significantly prolong OS and PFS compared with nivolumab alone or ipilimumab alone in patients with advanced melanoma. Subsequently, several studies have also investigated the efficacy of double immunotherapy in non-small cell lung cancer, renal cell carcinoma, colorectal cancer, liver cancer, biliary system tumours and so on.21–24 The combination of CTLA-4 and PD-1 blockers was shown to significantly enhance efficacy, and ipilimumab plus nivolumab was approved for the treatment of metastatic melanoma, advanced renal cell carcinoma and metastatic colorectal cancer with mismatch repair deficiency/high microsatellite instability (MMR/MSI-H) aberrations. Although with promising efficacy, the combination of CTLA-4 and PD-1 blockers significantly increased treatment-related grade 3–4 AEs. For example, in the CheckMate 067 Study, treatment-related grade 3–4 AEs were reported in 22% (70/313) of patients in the nivolumab alone group and 28% (86/311) of patients in the ipilimumab group, while in the nivolumab plus ipilimumab group, treatment-related grade 3–4 AEs were reported in 59% (185/313) of patients.
Cadonilimab (AK104) is a novel tetrameric form of PD-1/CTLA-4 bispecific antibody. In previous studies, cadonilimab has shown promising efficacy and superior safety compared with the coadministration of these individual agents in gastric, cervical, nasopharyngeal, liver and other cancers. In this study, we conduct a single-arm, phase II trial to investigate the efficacy and safety of chemotherapy plus cadonilimab as neoadjuvant treatment for locally advanced G/GOJ adenocarcinoma. To the best of our knowledge, this is the first study to explore the efficacy of neoadjuvant anti-PD-1/CTLA-4 immunotherapy combined with chemotherapy in G/GOJ cancer. However, the limitations of our study should also be addressed. First, this is a single-centre, single-arm, small-sample, phase II study. Large-cohort, randomised, phase III studies are required to further compare the efficacy and safety of neoadjuvant chemotherapy combined with cadonilimab versus standard chemotherapy in advanced/metastatic GC in the future. Second, the primary endpoint in the study is pCR rate, as less time will be required to obtain the primary results. Whether the high response rate of neoadjuvant therapy can translate into long-term survival benefits remain a focus of interest. Thus, the 2-year DFS rate and 2-year OS rate will also be evaluated to illustrate the survival benefit in the study. We hope this study will provide more evidence for neoadjuvant immunotherapy combined with chemotherapy in locally advanced G/GOJ adenocarcinoma and explore a series of predictive biomarkers of immunotherapy response.
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors J-KH, H-FG and P-FZ participated in the design of the study; P-FZ, WZ, XL and DH wrote the study protocol. P-FZ, KY, H-FG and J-KH are responsible for the conduct and coordination of the trial. All authors reviewed the manuscript draft and approved the final manuscript. J-KH and H-FG are the guarantors.
Funding This work was supported by the 135 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21006).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.