Article Text
Abstract
Introduction In light of the risks of over-reliance on opioid analgesia during recovery from rib fractures, there is increased interest in the efficacy of non-pharmacological approaches to pain management. This paper describes the protocol for a double-blind randomised controlled trial to evaluate the efficacy of an mHealth intervention for reducing pain intensity, pain-related distress and opioid use during early recovery from rib fractures.
Methods and analysis Adults (N=120) with isolated rib fractures will be recruited within 24 hours of admission to a large public hospital in Sydney, Australia (single site), and randomised (1:1 allocation) to an intervention or active control group. Clinicians, participants and statisticians will be blind to participants’ group allocation. The intervention (PainSupport) consists of a brief pain self-management educational video, followed by twice daily supportive Short Message Service (SMS) text messages for 14 days. Participants in the active control group receive the same video but not the supportive text messages. Participants in both groups continue to receive usual care throughout the trial. The primary outcome will be self-reported pain intensity on respiration measured using a Numerical Rating Scale. Secondary outcomes will include opioid use, pain-related distress, adherence to behavioural pain management strategies and the acceptability and feasibility of the intervention. Participants will complete questionnaires at baseline and then on days 1–7 and day 14 of the trial. A feedback survey will be completed at the end of the trial (day 15). Linear mixed models will be used to evaluate the main effect of the group on the primary and secondary outcomes and to explore differences between outcome trends recorded over the trial. Analyses will be based on the intention-to-treat principle to minimise bias secondary to missing data or dropouts.
Ethics and dissemination The study protocol has been reviewed and approved by the Northern Sydney Local Health District Human Research Ethics Committee (Australia). Informed consent is a requirement for participation in the study. Study results will be published in peer-reviewed journals and presented at scientific and professional meetings.
Trial registration number ACTRN12623000006640.
- Self-Management
- Pain management
- Psychosocial Intervention
- Trauma management
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Intervention and study measures codesigned with people who have lived experience of rib fractures.
Brief, validated and non-stigmatising measures of pain and concerns about pain have been selected to reduce patient burden.
The study targets patient participant feedback on the acceptability of the intervention and measures to further optimise the intervention and study design for future trials.
The active control group receives a brief version of the full intervention (fewer text messages); hence, there will be differences in frequency of contact with participants in the intervention and control groups.
Introduction
Background and rationale
Rib fractures require aggressive pain management, often including opioid therapy, to optimise pulmonary hygiene, respiration and minimise morbidity and mortality.1 However, higher doses of opioids during hospitalisation are associated with an increased risk of complications, including compromised ventilation2 and delayed discharge.3 Higher dose and longer use of opioids are associated with a greater risk of developing chronic pain4 or opioid dependency,5 as well as misuse and overdose.6 In view of growing concerns over the rapid escalation of prescription opioid-related deaths in Australia, reducing reliance on opioids for pain management has become a national health priority and is a key pillar of the National Strategic Action Plan for Pain Management.7
There is long-standing evidence for the opioid-sparing benefits of behavioural approaches to acute pain management.8 9 Behavioural interventions often include a combination of pain education and evidence-based skills to regulate thoughts, feelings and behavioural responses to unpleasant sensations. These interventions are thought to modulate pain intensity by reducing the threat of pain, emotional distress and physiological hyperarousal.10 11 Examples of behavioural pain management approaches include guided relaxation techniques, deep breathing exercises, self-compassion, emotional awareness and expression, positive social contact and connection with others, and pleasant activity scheduling. Many of these techniques, used alone or in combination, have been found to reduce pain intensity, distress and opioid use postsurgery.12–14
Despite consistent evidence for the benefits of adjunctive behavioural approaches to acute pain management, the costs and feasibility of integrating these interventions in practice—particularly in hospital settings—may be a barrier to implementation and effectiveness.15 Clinician-delivered behavioural pain self-management education in hospital settings can be costly: it is time-consuming,16 may place additional emotional burden on clinicians17 and requires additional clinical training.18 A second key challenge to effective implementation is that it may not be feasible for clinicians to engage patients fully with behavioural pain self-management in a ‘single session’ during an acute pain round. Pain impairs patients’ ability to attend to, comprehend and retain new information,19 and patients often find it difficult to sustain motivation to engage with behavioural pain self-management strategies without ongoing support.20 Hence, we must find ways not only to improve patient access to behavioural pain management education but also to provide patients with the ongoing support they need for continued engagement with behavioural pain management strategies.
Digital communication technologies including online videos, websites, apps and text messaging programmes offer low-cost, scalable, high-fidelity solutions to the problem of implementing and supporting ongoing patient engagement with behavioural pain management in hospital settings. In particular, a large body of research points to the feasibility, acceptability and efficacy of text messages as a means of engaging patients with a broad range of behavioural health interventions, including pain self-management.21–24 Text messages support mental and physical health by reminding patients to engage with behavioural strategies, by providing consistent healthcare contact, communication, emotional support and reassurance, and by reinforcing the importance of self-management.25 Receiving text messages in itself may have therapeutic value, as it is a subtle but familiar sign of social support.25 Indeed, pilot studies have found that receiving text messages containing reassuring messages has the effect of reducing pain during surgical procedures,26 reducing pain and anxiety associated with dental procedures27 and reducing chronic pain and pain interference.28
Objectives
We have codesigned29 and pilot tested,30 an mHealth intervention which delivers behavioural pain self-management education (via an online video) and ongoing support for using behavioural pain management strategies and reducing reliance on opioids, via twice daily text messages. Consistent with previous research, the text messages are designed to remind patients of behavioural pain self-management strategies, reinforce the value of behavioural pain self-management and reducing reliance on opioids and offer reassurance and validation. The aim of the current study is to evaluate the efficacy of this mHealth intervention for improving analgesia and recovery from rib fractures—a very painful condition in which the need to achieve effective pain management with the lowest possible dose of opioids is critical to patient outcomes. This study represents a first step towards utilisation of digital tools for supporting patient engagement with behavioural acute pain management in hospital settings more broadly.
Methods and analysis
This report is prepared according to the Standard Protocol Items: Recommendations for Interventional Trials (online supplemental appendix A).
Supplemental material
Patient and public involvement
The video and text messages used in this trial (online supplemental appendix B) were adapted from an intervention codesigned with people living with pain who were tapering off opioids as well as clinicians with expertise in pain management.29 We selected and adapted 40 text messages that were thought to be appropriate for people with rib fractures. A convenience sample (ie, people known to the research team from social networks) of five adults who had experienced rib fractures were asked to rate the text messages and video script for appropriateness and usefulness on a scale from 0 to 10. The 24 text messages with the highest ratings were included in the SMS library for this trial. The video script was edited in response to their feedback. In addition, the outcome measures, methods and study materials were informed by discussions with patients who have lived experience with rib fractures. We specifically sought input from patient advocates to ensure that the intervention and measures were not too burdensome. Patients/consumer partners will not be involved in the recruitment or conduct of the study, however, we are seeking feedback on the intervention from patient participants. This feedback will be used to make iterations to the intervention for further testing in a larger randomised controlled trial (RCT). Study participants are given the option of receiving feedback about the study findings, if they wish.
Supplemental material
Study design
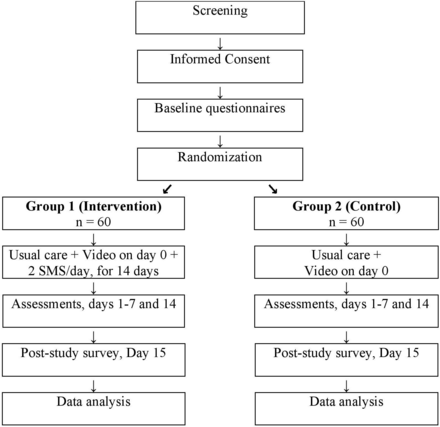
This is a double-blind RCT with two parallel arms (ratio 1:1). The study is designed to test the primary hypothesis which is the superiority of receiving (in addition to usual care) an educational video and twice daily SMS text messages versus video only in reducing pain intensity on respiration during early recovery from rib fractures (days 1–14) (figure 1).
Study flow diagram. SMS, Short Message Service text messages
There is currently no gold-standard ‘placebo’ control for digital health interventions and designing a sham condition is challenging in this field. Using an active control condition together with ‘limited disclosure’ will allow us to achieve blinding and investigate whether the observed effects can be attributed to a specific component of the digital support (ie, supportive text messages). Using an active control condition may also help to reduce the risk of dropouts and missing data. Moreover, mixed-method evaluations in this study will allow us to gather deep insight into the feasibility and acceptability of each component of the mHealth intervention, while repeated measures will provide the opportunity to better explore trajectories of change.
Setting and participants
The study will be conducted at the Royal North Shore Hospital in Sydney, Australia. 120 adult patients with isolated rib fractures within 1 day of admission to hospital will be recruited. Eligible patients will be identified by daily assessment of emergency admission notes and referrals to the acute pain service. To be eligible for the study, patients must be 18 years of age or older, admitted to hospital with isolated rib fracture/s in the last 24 hours, capable of reading and understanding basic English, capable of understanding study information and providing consent, have access to a mobile phone and able to use the phone during hospitalisation and be able and willing to complete survey measures on mobile phone. We will exclude those admitted to hospital with multiple injuries or comorbidities (eg, head, extremity, lung or abdominal trauma). We will also exclude patients who cannot use non-steroidal anti-inflammatory drugs and patients who are likely to receive regional anaesthesia or blockade.
Patients who are eligible to participate will be invited by a member of the acute and transitional pain service team (ie, either a pain nurse, registrar, or consultant) to participate in a ‘study examining factors influencing pain and recovery from rib fractures’. They will be told that the study will involve receiving text messages from the research team. Members of the acute and transitional pain service team will be responsible for checking eligibility either by consulting patient medical records or discussing with patients. Patients who express interest in the study will be provided with a QR code to scan with their mobile phones. The QR code will link patients to study information, consent forms (online supplemental appendix C), and (if they consent to participate) baseline measures formatted to be read on a mobile phone device (using the Qualtrics online survey platform). Hospital staff will not be notified of whether eligible patients choose to participate or not.
Supplemental material
Interventions
Intervention
The intervention is composed of a brief pain self-management video in combination with twice daily supportive text messages.
Pain self-management education video
The video will be approximately 10 min in duration and will include narrated, animated PowerPoint slides with information on behavioural pain management strategies (eg, relaxation techniques, thought management, distraction and social support seeking) as well as education about rib fractures (eg, reasons for pain, the importance of mobilising the lungs when breathing, timeline of recovery) and validation about the difficulty associated with breathing deeply with a rib fracture. Participants will receive the link to the educational video after randomisation (day 0) via their mobile phones. The video will also contain instructions for how to perform the pain on respiration test (used throughout the study to measure pain intensity) correctly.
Supportive text messaging
Participants will receive supportive text messages daily to reinforce the information provided in the video described above. Starting from day 1, participants will receive text messages (2 per day, 10:00 and 16:00 hours) for 14 days. Participants will receive all text messages in the same order. The text messages are designed to remind participants of pain self-management strategies and the value of using them (eg, ‘Having a chat with a friend or family member—even if by text or phone—is a great way to distract you from the feeling of muscle spasms’), reinforce the importance of optimising inhalation to mobilise the lungs (eg, ‘Don’t forget to keep up with your deep breathing to keep your lungs healthy’) and provide patients with validation and reassurance (eg, ‘You may be feeling pain and discomfort during your recovery. Remember this is part of the recovery process for everyone’).
The messages sent to the participants are standardised in their content and delivery (by day of study and by the time of day). All text messages are short and use simple language. Participants’ first names are used in a selection of messages to increase engagement (eg, ‘Hi John’). Messages will be sent over Australian telephone networks at no cost to individual participants. Messages will be sent between 9:00 and 17:00 hours (local time).
Participants will be informed that the text messages are designed to be one-way and that responses to the text messages will not be monitored by the research team in real time. However, they can cease receiving text messages by contacting the chief investigator, who will give participants the option of withdrawing from the study if they wish. If withdrawing from the study, no further assessment will be sent to the participant. A sensitivity analysis will be conducted to account for adherence (ie, excluding participants who stop receiving text messages).
Active control
Participants in the active control group will receive the same pain self-management video as participants in the intervention group. The active control group will not receive additional supportive text messages. However, they will receive text messages containing links to brief online surveys containing study outcome measures (at 15:00 hours each day (as will the intervention group). Consequently, the intervention and active control groups will both expect and receive text messages supporting double blinding.
Participants in both groups will continue to receive usual care throughout the study period. Usual care in this context is multidisciplinary with a focus on maximising non-opioid analgesia while encouraging pulmonary hygiene. It may include incentive spirometry, high-flow nasal prong oxygen and/or multimodal analgesia (including patient-controlled analgesia).
Outcomes
To minimise participant burden, we will use short-form versions of validated questionnaires as described at the times specified in online supplemental table 1.
Supplemental material
Pain intensity on respiration (primary outcome)
Pain intensity was assessed daily for 7 days after study enrolment (days 1–7) and again on day 14. Average pain reported over the course of days 1–7 will be compared between groups. Participants will be asked to take a deep breath and record their pain using an 11-point Numerical Rating Scale (0=no pain, 10=worst pain imaginable).31 Assessment of pain intensity using a numerical rating scale has been supported in prior studies.32
Pain-related distress (secondary outcome)
Pain-related distress was assessed daily for 7 days after study enrolment (days 1–7) and again on day 14 using the 2-item Concerns About Pain Scale.33 Pain catastrophising is a term which captures patterns of negative cognition (worry, concern) and emotion (distress) in the context of actual or anticipated pain. Because the term ‘catastrophising’ is considered stigmatising by people with chronic pain,34 we will use the phrase ‘pain-related distress’. We will modify the instructions for this scale from ‘in the past 7 days’ to ‘in the past 24 hours’ to better fit with the acute pain context.
Opioid use (secondary outcome)
Opioid use was assessed daily during hospitalisation (extracted from health records) and again on day 14 (self-reported use over the previous 72 hours). Opioid use will be converted to an oral morphine equivalent daily dose using the ANZCA opioid converter (http://www.opioidcalculator.com.au). Opioids prescribed at discharge will be retrieved from medical records.
Self-reported adherence to behavioural pain management strategies
Self-reported adherence was assessed on days 7 and 14 using a single self-report item (6-point Likert scale): ‘How often are you using the strategies you learned in the video that you watched when you enrolled in the study to help you to manage your pain?’ (1=not at all, 2=a couple of times in the week, 3=every couple of days, 4=once a day, 5=a couple of times a day, 6=several times a day).
Acceptability of intervention components
Acceptability was assessed using a survey at the end of the study period (day 15). The survey design was based on previous studies conducted by our research team and contains Likert scales and open-ended questions about the usefulness, readability and acceptability of the digital support.30
Feasibility of intervention components
Feasibility was evaluated at the end of the study according to the number of messages delivered/not delivered which will be recorded automatically by the text message system. We will also report on (1) the number of candidate participants who were excluded and reasons for exclusion; (2) the number of eligible candidates who chose not to enter the trial; (3) all protocol deviations that may impact the interpretation of the trial results; (4) the number of withdrawals from each treatment group, including patients lost to follow-up and the reasons for withdrawals if known and (5) the types, rates and reasons for non-adherence with treatment in each treatment group (eg, not watching the video). This information will either be recorded by the hospital staff handling recruitment or the chief investigator.
Engagement with the video
Engagement was assessed using a study-specific questionnaire shortly after participants received the link to the video (day 0). Participants will asked to confirm they have watched the video by answering two questions about the content of the video.
Engagement with the text messages
On day 15, participants in the intervention group will be asked whether or not they read the text messages they were sent during the study.
Participant characteristics
Psychosocial characteristics including history of pain and medication use, cause of injury, context of injury, perceived fault of injury, history of rib fractures and social support will be recorded at the beginning of the study (day 0), as will baseline pain intensity and pain-related distress. Socioeconomic characteristics including education, language spoken at home and place of usual residence (postcode) will be self-reported at the end of the study (day 15) to reduce the burden associated with the baseline survey. Clinical data (number of rib fractures, location of rib fractures, comorbidities, pain management plan, date and time of admission, age and gender) will be extracted from hospital records.
Enrolment and randomisation
Participants who have provided informed consent will automatically be enrolled in the study. Once enrolled, participants will be randomised (1:1 allocation) to either the intervention (video plus supportive text messages) or active control (video alone) group in blocks of six (figure 1). Block randomisation will be used to ensure the study groups are balanced. A research team member will be responsible for randomising patients and programming text messages for delivery. The team member who is aware of the group allocation will not be involved in recruitment, data collection or analysis after randomisation to prevent bias. Once the participant has been randomised, the member will then programme the text messages accordingly with delivery starting the following morning.
Blinding arrangements
Participants, their clinicians, research team members (except the member who manages text messaging and the Chief Investigator who will monitor data collection and manage withdrawal from the study) and statisticians are all blinded to the group allocation.
Participants in both the intervention and active control groups receive their treatments online, via their mobile phone devices and all participant-reported data are collected online. There is limited in-person contact with clinicians involved in the study and participants will be asked not to talk about the content of the text messages with doctors or other staff at the hospital.
The statistician will be blinded to the group allocation. At the end of the study, a group variable will be added to the study database and each group will be labelled as A or B before it is sent to the statistician for statistical analyses. Groups will be unblinded after all analyses comparing groups are complete.
Participants will be informed that they will be randomly allocated into two groups. They will be informed that both groups will receive digital health support. They will be informed that the study aims to evaluate whether digital supports can improve health outcomes in patients with rib fractures. This ‘limited disclosure’ of the between-group difference (ie, study hypothesis) is required to maintain double-blinding and to achieve the main objective of the research in evaluating the efficacy of receiving daily text messages. This method does not involve deception and is recommended for ‘Improving Blinding Integrity and Reporting in Psychotherapy Trials’35 and has previously been used in similar studies.30 36
Data collection
Outcome measures will be completed online via Qualtrics software. The links to the Qualtrics surveys will be sent to participant’s mobile phones. If participants do not respond to measures sent by SMS on days 14 and 15, they will be sent a reminder text message.
Statistical methods
Sample size
Sample size was calculated using the online app GLIMMPSE (General Linear Mixed Model Power and Sample Size V.3.0) for testing the overall main effect (ie, group) with 7 days of repeated measures postintervention (days 1–7). The study was powered to test for mean differences in pain intensity on respiration (primary outcome). We set a power threshold of 0.8, a two-tailed alpha of 0.05, SD of pain at 2.5 and SD ratio at 1.5 over 7 days. Accordingly, we estimate the need for 51 patients per group to detect a 1.3 (out of 10) difference, in overall, in pain intensity. This is the minimum clinically important difference for average pain levels (4–7 out of 10) in acute pain.37 With 15% estimated dropouts, we will recruit 120 patients.
Data analysis
All analyses will be blinded to the group status. Descriptive statistics of demographics and other baseline variables will be reported. To address the study primary hypothesis, which is an overall lower pain intensity 7 days after randomisation, a mixed model analysis will be used, evaluating the main effect of the group (intervention vs active control). Model statement (eg, whether to include day 0 pain as a covariate in the model) and parameters (eg, covariance structure) will be determined based on the fit statistics and distribution of residuals. Mixed model will also be used for comparing opioid use and distress (measured over 7 days) and adherence (measured at days 7 and 14) between the two groups. Data will be transformed to approximate residuals to normality if required. A planned contrast will be applied for comparing pain at day 14 between the groups while including pain at day 0 as a covariate in the model. Linear mixed models will be used to explore the differences in outcome trends recorded over the 7 days after randomisation. Other outcomes will be compared between the two groups using independent t-test (or Mann-Whitney U test). Exploratory analyses will investigate the correlation between baseline characteristics (gender, age, number of rib fractures, location of rib fractures, history of long-term opioid use) and pain intensity days 1–7 and day 14. We will also explore the relationship between baseline sociodemographic characteristics and in-patient opioid prescribing and opioid prescribing on discharge. Supplementary analyses will include these baseline characteristics as covariates in additional analyses if they are found to be predictors of pain outcomes. All analyses will be conducted by using SAS software (V.9.4) and based on the intention-to-treat principle (all randomised participants will be included in the analyses) to minimise bias secondary to missing data or dropouts. Where applicable, results will be reported with 95% CIs, with an alpha of 0.05 used to establish statistical significance.
Recruitment timeline
Recruitment for this study will begin in October 2024 and continue through October 2025.
Ethics and dissemination
The study was granted ethical approval from the Human Research Ethics Committee of the Northern Sydney Local Health District (Protocol Version 2, dated 25 May 2023, Reference: 2023/ETH01114, online supplemental appendix D). Informed consent is a requirement for participation in the study: all participants are required to be capable of reading English, understanding consent forms and providing consent. Deidentified datasets will be made available from the corresponding author on reasonable request. The trial results will be published in peer-reviewed academic journals and presented at scientific and professional meetings. Authorship will comply with International Committee of Medical Journal Editors guidelines.
Supplemental material
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
X @drashtonjames, @AmyMcNeilage
Contributors Conceptualisation: CA-J, DF, MD, PG and AG. Trial registration and ethical approval: CA-J and AGM. Drafting protocol manuscript: CA-J and AGM. Reviewing and editing: DF, MD, PG and AG. Project administration: CA-J, AGM and AG. Funding acquisition: CA-J, DF, MD, PG and AG. CA-J is the guarantor.
Funding The project was funded by a Ramsay Foundation Philanthropic Grant (grant award number: N/A) and the trial sponsor is Northern Sydney Local Health District.
Disclaimer The funder and sponsor had no role in design of the study or preparation of the protocol.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.