Article Text
Abstract
Objectives The aim of this study was to evaluate the cost-effectiveness of trifluridine/tipiracil (FTD/TPI) for heavily pretreated metastatic gastric cancer from the perspective of the Chinese healthcare system.
Designs Based on the overall survival and progression-free survival (PFS) data from the Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS) trial (NCT02500043), a three-state Markov model (PFS, progressed disease and death) was constructed to analyse the cost-effectiveness of FTD/TPI compared with the placebo in heavily pretreated metastatic gastric cancer. Cost and utility were from pricing records and the literature. The model was simulated for 5 years with monthly cycles. Costs and health outcomes were discounted by 5%. We then conducted sensitivity analyses to evaluate the robustness of the parameters. The model results were from the Chinese healthcare system.
Outcome measures The output results were the quality-adjusted life years (QALYs) and incremental cost-effectiveness ratio (ICER).
Results According to the model results, FTD/TPI generated an additional cost of US$26 855.66 and 0.88 QALYs compared with the placebo. ICER of FTD/TPI compared with the placebo was US$30 494.89 per QALY. Sensitivity analyses revealed that the utility value of the PFS stage and FTD/TPI adverse event costs were the main influencing parameters, and the results were stable. At a threshold of three times per capita gross domestic product of China (US$35 559.34 in 2022), the probability of FTD/TPI being cost-effective compared with placebo was 99.2%.
Conclusion From the perspective of the Chinese healthcare system, FTD/TPI is a more cost-effective option compared with the placebo for the treatment of heavily pretreated metastatic gastric cancer in patients who have received at least two prior advanced treatment regimens.
Trial registration number The Chinese population registered in the Chinese Clinical Trial Registry (ChiCTR2400080940) and clinical trial (NCT05029102).
- Health Economics
- Gastrointestinal tumours
- China
Data availability statement
Data are available in a public, open access repository. All data relevant to the study are included in the article or uploaded as supplementary information. The original data used in this article comes from the TAGS clinical trial (NCT02500043). If you need to query the specific original data, please contact the original author.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study used a Markov model to analyse the cost-effectiveness of trifluridine/tipiracil (FTD/TPI) for the treatment of heavily pretreated metastatic gastric cancer in China.
The cost of adverse events (AEs) only considers the severe AEs of grade 3 and above and does not consider all AEs.
Based on the population distribution of TAGS participants, the majority are Europeans. This limitation may introduce biases in assessing real-world clinical efficacy in China.
The model uncertainty concerning short-term survival rates is small owing to the good fitness of the model. But the long-term benefits of FTD/TPI require further analysis.
Introduction
According to the latest statistics from the WHO in 2022, gastric cancer is the fourth leading cause of cancer-related deaths worldwide.1 About 1.2 million new cases of gastric cancer occur worldwide every year, with China accounting for approximately 40% of them.2 Many patients are in an advanced state of cancer cell metastasis when gastric cancer is discovered,3 and treatment is usually limited to palliative chemotherapy because of poor expected results.
At present, the guidelines of the Chinese Society of Clinical Oncology (CSCO) and the National Comprehensive Cancer Network (NCCN) recommend several treatment options for metastatic gastric cancer, including combination chemotherapy and single-agent chemotherapy. As a result of the generally poor physical condition of patients with advanced third-line gastric cancer, the proportion of patients who can receive combination third-line chemotherapy is extremely low, and single-agent treatment is mainly used. Patients who have received these chemotherapy treatments before receiving trifluridine/tipiracil (FTD/TPI) treatment have reported unsatisfactory results, and the cost is also high. According to Iqvia Holdings Inc (IQVIA),4 global expenditure on oncology drugs will continue to increase at a double-digit rate. Therefore, a cost-effective and efficient treatment for gastric cancer remains a global challenge.
In 2019, the European Commission and the Food and Drug Administration (FDA) approved FTD/TPI for the treatment of adult patients with metastatic gastric cancer who have received at least two prior systemic treatment regimens to manage advanced disease. FTD/TPI is a novel oral cytotoxic chemotherapy consisting of a thymidine-based nucleoside analogue, trifluridine and thymidine.5 As the main active ingredient, trifluridine inhibits cell proliferation by direct insertion into DNA after phosphorylation, leading to DNA dysfunction and cell death.5 6 In addition to its anti-tumour role when combined with trifluridine to form FTD/TPI, it prevents the rapid degradation of trifluridine, allowing for the maintenance of adequate plasma levels of the active drug.6 7
In 2015, FTD/TPI was first approved by FDA and expanded globally including the European Union and China for the treatment of patients with heavily pretreated metastatic colorectal cancer.8 In 2021, FTD/TPI generic tablets were first approved for marketing by the National Medical Products Administration.9 This move greatly reduced the cost of using FTD/TPI in China. FTD/TPI has been approved for metastatic colorectal cancer in the current CSCO guidelines, but it has not been approved for metastatic gastric cancer. There is currently no cost-effectiveness analysis evaluating FTD/TPI for the treatment of a significant number of patients with heavily pretreated metastatic gastric cancer from the perspective of the Chinese healthcare system. Therefore, this study conducted a cost-effectiveness analysis of FTD/TPI for treating a large number of patients with heavily pretreated metastatic gastric cancer from the perspective of the Chinese healthcare system. The results of this study could provide clinicians and payers with economic evidence to consider incorporating FTD/TPI into Chinese guidelines for the diagnosis and treatment of heavily pretreated metastatic gastric cancer and guide the pricing of the originator for metastatic colorectal cancer and other indications.
Methods
Patients
The data were selected from the TAGS trial10 (NCT02500043, Taiho Oncology, Inc.). This work is a phase III study with randomised, double-blind, placebo-controlled and multi-national cases. The study began in July 2015 and concluded in September 2021, including 17 countries and involving 110 academic hospitals, evaluating the efficacy and safety of FTD/TPI plus best support care (BSC) versus placebo plus BSC in participants with heavily pretreated metastatic gastric cancer. After screening and excluding certain cases, a total of 507 patients participated in this clinical trial. These patients had received at least two treatments for advanced gastric cancer before. Eligible patients were randomly assigned in a 2:1 ratio to either the FTD/TPI group (337 patients) or the placebo group (170 patients). The primary endpoint of the study was overall survival (OS), while the secondary endpoint was progression-free survival (PFS). The objective of TAGS trial was to investigate whether the quality of life (QoL) of patients could be maximised without the influence of anti-tumour factors. Patients or members of the public were not involved in the design of this study.
Treatment
Participants received 35 mg/m2 FTD/TPI tablets orally two times per day for 5 days per week (from days 1 to 5 and days 8 to 12) for 2 weeks, followed by 14 days of rest in each 28-day cycle along with BSC until the patient met the drug suspension standard (including participant withdrawal, disease progress, irreversible treatment related to four non-haematological events, doctor’s decision, participants who are pregnant or death).
Model structure
A three-state Markov model was constructed to simulate the cost-effectiveness differences between FTD/TPI plus BSC and placebo plus BSC for treating heavily pretreated metastatic gastric cancer using TreeagePro 2019, including PFS, progressed disease (PD) and death (D) states. Patients entered this model in the PFS state and could not return to the previous state after entering PD. The entry point into the model for patients in this study was established at the average age of participants in the TAGS trial (62.5 years). According to the model, the Markov cycle was 28 days. Given that 99.9% of patients entered the D state after 60 model cycles, and the overall 5-year survival rate for progressive gastric cancer is only 35.1%,11 the model was limited to a duration of 5 years. A discount rate of 5% for both cost and utility values is recommended according to the Chinese Pharmaceutical Economics Evaluation Guide (2020).12
Transition probability
The OS and PFS curves were derived from the TAGS trial. The GetData Graph Digitizer software was used to collect data points from OS and PFS survival curves. The data were then cleaned and converted into a format suitable for survival analysis. The data were analysed by Kaplan-Meier analysis through R4.2.0 software, and survival curve extrapolation was conducted using the standard parametric model with Weibull, gamma, lognormal, log-logistic and exponential distributions (table 1). According to the Akaike information criterion (AIC) and Bayesian information criterion (BIC), the smaller the AIC and BIC values, the better the fit.13 Therefore, the log-normal distribution was selected as the optimal distribution based on AIC and BIC, along with a visual inspection of the FTD/TPI and placebo data. We included both the original survival curves and the simulated survival curves (table 2). The parameters μ and θ for each group of OS and PFS curve parameters were obtained to calculate the transition probability from PFS to PFS (PFTF). Assuming that the transition probability from PFS to D (PFTD) corresponds to a per capita mortality rate of 7.37‰ in 2022,14 PFS to PD (PFTP)=1-PFTF-PFTD. The OS curve parameters can be used to determine the transition probability from the survival state to the survival state (PSTS) and the survival state to D (PSTD)=1 PSTS. According to Zhou15 the transition probability from PD to PD (PPTP) should be adjusted. Therefore, PPTP = ([nPFS+nPD]×PSTS-nPFS×PFTF-NPFS×PFTD)/nPD, PPTD=1 PPTP. Among them, nPFS and nPD are the number of patients in the PFS and PD states in the previous cycle, respectively.
Progression-free survival and overall survival data fit results
Comparison of simulated survival curves with original survival curves
Costs
From the perspective of the Chinese healthcare system, this study determined and analysed the following direct costs (US dollar (US$); US$1=¥7.23 (2023)): drug costs, administration costs, adverse event costs, imaging costs and BSC costs. All costs were discounted by 5%. We assumed that FTD/TPI was used continuously until the patients met the drug suspension standard. The cost of the drugs was sourced from Yaozhi.com,16 with the median bid price of the drugs in each province considered as the cost of the drugs (table 3). In the TAGS trial, the patient’s dosage was determined based on their body surface area, which was calculated to be 1.60 m2 using the Stevenson formula and the average height and weight of individuals in China. Six provinces in China,17–22 including Hunan, Henan, Jiangsu, Anhui, Shaanxi and Shandong, were selected to estimate the administration cost, imaging cost and BSC cost based on the prices listed in the price catalogue of medical services of the Medical Insurance Bureau of each province. Administration costs include the expenses associated with patient accommodation during hospitalisation, nursing fees and routine examinations, such as blood routine, urine routine and stool routine. Imaging tests such as CT, MRI, positron emission tomography CT and radiography were conducted every two cycles. The cost of BSC was estimated based on the potential monitoring components of palliative care outlined in the 2022 gastric cancer treatment guidelines.23 The estimation was conducted in conjunction with the median prices listed in the medical service price item catalogue of the six provinces.
Cost and utility value parameters
We assumed that patients would receive BSC treatment after PD. Treatment options for adverse events were derived from the NCCN guidelines.24 The cost of adverse events treatment was estimated based on the medical service price item catalogue of the six provinces and Yaozhi.com. The cost of adverse events (AEs) only considers severe AEs of grade 3 and above (grade ≥3), including neutropenia (34%), anaemia (19%) and leucopenia (9%) in the FTD/TPI group, as well as bellyache (9%) and anaemia (8%) in the placebo group.
Utility
In 2021, the health-related QoL in the TAGS study was evaluated using the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire. Patients completed the EORTC QLQ-C30 questionnaire within 7 days prior to randomisation, before dosing on day 1 of at least two treatment cycles and during the safety follow-up 30 days after the last dose (if not conducted within the previous 4 weeks). To obtain EQ-5D utility weights to populate the model, Hamerton et al 25 used a published algorithm by Kontodimopoulos et al to map the scores from the EORTC QLQ-C30. The resulting utility values applied within the model were 0.764 for PFS and 0.652 for PD.
Model-based results
The output results were the quality-adjusted life years (QALYs) and incremental cost-effectiveness ratio (ICER). Willing-to-pay (WTP) is suggested as three times per capita GDP in China. When the ICER value was less than WTP(US$35 559.34), FTD/TPI was found to be more cost-effective than the placebo. For a health intervention to be considered cost-effective, a WTP threshold of US$35 559.34 per QALY was used in the current analysis. Published studies reported that a treatment should be considered cost-effective if the ICER is between one and three times the GDP per capita of that country and a treatment is considered highly cost-effective at less than one time the GDP per capita.26–28
Sensitivity analysis
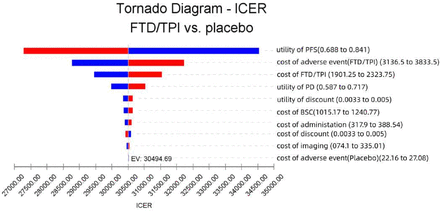
To evaluate the robustness of the Markov model, we performed one-way sensitivity analysis and probabilistic sensitivity analysis (PSA) to assess the impact of different parameters on the basic analysis results. The range of each variable was floated by 10% based on the base value. Sensitivity analysis was performed using TreeagePro 2019 software. One-way sensitivity analysis results are typically presented in the form of a tornado diagram, which can reflect the size of multiple uncertain factors on the outcome.25 In the PSA, we set the cost parameter as the gamma distribution and the utility value as the beta distribution, extracting values from the corresponding distribution for 1000 Monte Carlo simulations, and the results were presented as a cost-effectiveness acceptability curve.
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, report or dissemination plans of this research.
Result
Base-case analysis
According to lognormal fitting, the median survival of OS for FTD/TPI and placebo was 6 and 3.6 months, and the median survival for PFS for FTD/TPI and placebo was 2 and 1.8 months, respectively. The simulated PFS curve and OS curve closely resembled the original data (the median survival of OS for FTD/TPI and the placebo was 5.7 and 3.6 months; the median survival for PFS for FTD/TPI and the placebo was 2 and 1.8 months), indicating an acceptable and reasonable fit.
Based on the Markov model, from the perspective of Chinese healthcare system (table 4), the total treatment cost for FTD/TPI was US$32 234.26, while that for placebo was US$5378.6. Compared with the placebo, the ICER value for FTD/TPI was US$30 494.89 per QALY gained, with an incremental cost of US$26 855.66 and an incremental QALY of 0.88, which was lower than WTP. Therefore, compared with the placebo, FTD/TPI is a cost-effective treatment option for heavily pretreated metastatic gastric cancer.
Results of the base-case analysis
Sensitivity analysis
One-way sensitivity analysis
The tornado diagram in figure 1 shows the rank order of the impact degree of each variable on the results. The utility value of the PFS stage had the greatest effect on the ICER value, followed by the AE cost of FTD/TPI. FTD/TPI remained a cost-effective treatment given that the ICER per QALY gained remained below the threshold of US$35 559.34 per QALY gained. Individual parameter changes may slightly alter the overall value associated with the treatment, but they do not change the ICER-based conclusion of FTD/TPI in the treatment of heavily pretreated metastatic gastric cancer.
Cost and utility value range fluctuation ±10% under the order factor sensitivity analysis tornado diagram. BSC, best support care; EV, expected value; FTD/TPI, trifluridine/tipiracil; ICER, incremental cost-effectiveness ratio; PD, progressed disease; PFS, progression-free survival.
Probabilistic sensitivity analysis
According to the cost-effectiveness acceptability curve (figure 2), when WTP increased within the range of 1–3 times threshold (US$11 860–US$35 559.34) of the GDP, the FTD/TPI showed an increase in economic feasibility. The analysis showed that FTD/TPI had a probability of 99.2% of being a cost-effective option compared with the placebo, at a WTP threshold of US$35 559.34. Thus, FTD/TPI treatment for heavily pretreated metastatic gastric cancer is a cost-effective option. At a threshold of 2.5 times per capita for GDP, FTD/TPI’s cost-effectiveness probability dropped to 34.1%, whereas that of the placebo increased to 65.9%.
Cost-effectiveness acceptability curve for probabilistic sensitivity analysis. CE, cost-effectiveness; FTD/TPI. trifluridine/tipiracil.
Discussion
Recent research has shown that FTD/TPI has an obvious effect on heavily pretreated metastatic gastric cancer. Although it has not yet been included in the CSCO guidelines, with the discovery of the value of FTD/TPI in the treatment of heavily pretreated metastatic gastric cancer, the use of FTD/TPI can be considered for the treatment of patients in the clinic. This has significant implications for the physiological and psychological well-being of patients and has significant social implications.
In this study, the TAGS trial showed that FTD/TPI provided a significant survival benefit for patients with heavily pretreated gastric cancer compared with the placebo. We aimed to conduct a Markov model analysis of FTD/TPI compared with the placebo in patients with heavily pretreated metastatic gastric cancer from the perspective of the Chinese healthcare system. According to our analysis, FTD/TPI costs US$26 855.66 more than the placebo and provided an additional 0.88 QALYs, resulting in an ICER of US$30 494.89 per QALY, which was below the defined WTP of US$35 559.34 per QALY gained. The analysis showed that FTD/TPI had a probability of 99.2% of being a cost-effective option compared with the placebo at a WTP threshold of US$35 559.34. Therefore, from the perspective of the Chinese healthcare system, FTD/TPI treatment for heavily pretreated metastatic gastric cancer is cost-effective compared with placebo.
Several international publications currently use data from the TAGS trial to compare the cost-effectiveness of FTD/TPI in patients with heavily pretreated metastatic gastric cancer. Takushima et al 29 used a partitioned survival model (PSM) to estimate the cost-effectiveness of FTD/TPI versus nivolumab from the perspective of the Japanese public healthcare payer. According to their results, the ICER of nivolumab and FTD/TPI is ¥32 352 489/QALYs, and the WTP threshold is ¥7 500 000. Therefore, the analysis of FTD/TPI from the Japanese public healthcare payment perspective shows that it is more cost-effective than nivolumab. However, as a result of differences between nivolumab and placebo, we could not compare the results of Takushima with this study due to the different factors involved. In Britain, Hamerton used a PSM to compare FTD/TPI with BSC in the UK.25 A lognormal distribution to fit OS and a generalised gamma model to fit PFS and time-to-treatment-discontinuation were employed. According to the study results, FTD/TPI was associated with an ICER of £37 907 per QALY gained compared with BSC. Therefore, FTD/TPI is a cost-effective treatment for patients with heavily pretreated metastatic gastric cancer from a UK perspective. In Greece, Tzanetakos et al 30 analysed the TAGS data through a PSM from the perspective of the Greek public payer. They reported an ICER of €47 144 per QALY gained and €28 112 per QALY gained compared with BSC. Therefore, FTD/TPI was estimated to be a cost-effective treatment option for eligible third-line treatment of patients with metastatic gastric cancer in Greece. The results of both are consistent with the results of our study.
Overall, the published literature supported the findings of the present analysis, except for the study by Zhou t al. 31 They developed a Markov model to assess the cost-effectiveness of FTD/TPI from the perspective of the US payer. According to the results, compared with the placebo, the increase in FTD/TPI is 0.06 QALYs, and the ICER value is US$986 333, which is far beyond their WTP threshold (US$50 000–US$150 000). They found that FTD/TPI does not provide cost benefits from the perspective of US payers. Their results were not consistent with our study, which may be attributed to the varying prices of FTD/TPI in different countries. Chinese generic drugs hold a dominant position in the domestic drug market. The emergence of generic drugs can reduce drug prices and increase drug accessibility.32 Domestic generic drug manufacturers have implemented Porter’s generic strategies to adjust prices, resulting in an average profit margin of only 5% to 10% for generic drugs in China, which is significantly lower than the average profit margin of international generic drugs (30% to 60%).33 Therefore, the cost of generic drugs in China is generally lower compared with the cost of foreign drugs.
However, our study also had some limitations. First, the model uncertainty concerning the original survival rates was small due to the excellent fit of the model. The long-term benefits of FTD/TPI require more analysis. Second, only considering the cost of AEs at level three or above, without taking into account all AEs, may lead to bias in the data. Third, based on the population distribution of participants in the TAGS trial, the majority were Europeans. This could introduce biases in real-world clinical efficacy in China, potentially impacting the trial’s generalisability. Fortunately, exploratory studies on FTD/TPI for heavily pretreated metastatic gastric cancer in the Chinese population registered in the Chinese Clinical Trial Registry (ChiCTR2400080940) and clinical trial (NCT05029102) are currently underway. As data are continually updated, this study will also be updated.
Conclusion
In summary, from the perspective of the Chinese healthcare system, FTD/TPI is a cost-effective choice for heavily pretreated metastatic gastric cancer. The results of this study could provide clinicians and payers with economic evidence to consider incorporating FTD/TPI into CSCO guidelines for the diagnosis and treatment of heavily pretreated metastatic gastric cancer and guide the pricing of the originator for metastatic colorectal cancer and other indications.
Data availability statement
Data are available in a public, open access repository. All data relevant to the study are included in the article or uploaded as supplementary information. The original data used in this article comes from the TAGS clinical trial (NCT02500043). If you need to query the specific original data, please contact the original author.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
Acknowledgments
The author would like to thank the financial support of The University Synergy Innovation Program of Anhui Province (grant number: GXXT2021-068), and we thank the associate editor and the reviewers for their useful feedback that improved this paper.
Footnotes
Contributors TY and RX are joint first authors. YZ, JD and YW were involved in the data acquisition. TY, RX and YZ were involved in the statistical analysis. JD, YW and XX were involved in the analysis and interpretation of the data. TY, RX, YZ and JD were involved in the critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript. TY and RX are the study guarantors.
Funding This work was supported by The University Synergy Innovation Program of Anhui Province (grant number: GXXT-2021-068).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, report or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.