Article Text
Abstract
Introduction Migraine is a widespread neurological disorder characterised by recurrent moderate-to-severe headaches. These headaches can seriously affect patients’ daily life and work, especially during acute attacks when patients often need immediate pain relief. This study aims to assess the immediate analgesic effect of acupuncture for 10 min during acute migraine attacks.
Methods and analysis The study will randomly divide 80 participants into either the acupuncture group or the sham acupuncture group with an allocation ratio of 1:1. Each group will receive 10 min of treatment, and the post-treatment evaluation will be performed after 0, 0–2, 4, 6, 8 and 10 min of acupuncture. The primary outcome is the pain Visual Analogue Scale (VAS) score assessed before and after treatment at 10 min. Additionally, secondary outcomes include the pain VAS score assessed at 0–2, 4, 6 and 8 min, blinding assessment and treatment effectiveness expectations scale. Data will be collected at baseline time and the end of treatment (after 10 min). Adverse events during each treatment period will be collected and recorded.
Ethics and dissemination Ethics approval was obtained from the Ethics Committee of the Second Affiliated Hospital of Yunnan University of Chinese Medicine (2022–008). All participants will provide written informed consent before randomisation. The results of this study will be published in a peer-reviewed journal and presented at conferences.
Trial registration number Chinese Clinical Trial Registration Center (ChiCTR2200066976).
- Pain management
- COMPLEMENTARY MEDICINE
- Migraine
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This study is the first randomised controlled study to evaluate the immediate analgesia efficacy of acupuncture for acute migraine attacks.
The results of this study will provide reliable clinical evidence for the use of acupuncture in the treatment of acute migraine attacks.
No positive drug control group is set-up in the study.
No follow-up is set for this study.
This study is not a multicentre study.
Introduction
Migraine is a widespread neurological disorder characterised by moderate-to-severe throbbing headache, often accompanied by photophobia, phonophobia, nausea and vomiting, which can seriously affect patients’ daily life and work during acute attacks.1 Among them, migraine without aura (MwoA) is the most common type of migraine, accounting for about 80% of migraine.2 Studies have shown that the incidence of migraine in the acute phase is 17.4% in women and 5.7% in men, and the incidence of women is about two to three times higher than that of men.3 4 In addition, the disability-adjusted life years and the economic burden of migraine are increasing year by year. At present, migraine remains the second leading cause of disability in the world and ranks first among young women.5 According to the Global Burden of Disease Report 2019, migraine accounts for 88.2% of the burden of disease for headache and causes approximately 42.1 million years of disability years of life lost (YLDs) per year and 4.8% of total YLDs.6 There have been more than 1 billion cases of migraine worldwide, with an annual prevalence as high as 15%, with the highest prevalence in European countries at 35%, in the USA at 12–13% and in East Asian countries at 25–35%.7 And in China, the prevalence is relatively low at 9.3%, but the absolute number of cases is still large due to the large population base.8
At present, western medicine is mainly used for pain relief in the acute phase of migraine with non-specific therapeutic drugs (non-steroidal anti-inflammatory drugs) and specific therapeutic drugs (triptans and ergot drugs), which have significant clinical treatment effects, but long-term use has drug resistance and certain side effects.9 Acupuncture, as a safe and effective non-pharmacological treatment, is gaining increasing interest. Studies have shown that acupuncture is more effective in the treatment of MwoA than sham acupuncture and conventional treatment, that it significantly reduces the frequency of migraine attacks and that it has a faster onset of action compared with oral medication.10 Acupuncture shows a promising trend in effectively reducing the frequency of migraine attacks. One study in comparing the efficacy of acupuncture with sham acupuncture and waiting for treatment of migraine found that the acupuncture group significantly reduced the frequency of migraine attacks, with a mean reduction in the frequency of attacks of 3.2 (2.1).11 Another study has demonstrated that the frequency would be reduced to 3.5 days in the acupuncture group relative to the sham and preventive medicine groups.12 These results indicate that acupuncture has the potential to be an effective method of controlling the frequent occurrence of migraine attacks.
However, migraine is a chronic disease, which is difficult to completely cure and requires long-term maintenance treatment. Patients in the acute attack stage urgently need immediate pain relief. Studies have also shown that acupuncture is superior in relieving acute pain.13 Our team has shown in a previous study that acupuncture is effective for immediate analgesia in acute migraine attacks, but with limitations such as a small sample size and no control group.14 Further research is needed to support the immediate analgesic effect of acupuncture in migraine.
Objectives
This study aims to evaluate the immediate analgesic effect of acupuncture for acute migraine attacks.
Methods and analyses
The study protocol was designed in accordance with standard protocol project design: Recommendations in the Guidelines for Interventional Trials15 (online supplemental file 1). The study protocol was approved by the Medical Ethics Committee of the Second Affiliated Hospital of Yunnan University of Chinese Medicine (2022–2023).
Supplemental material
Study design and setting
This randomised and sham-controlled clinical trial was designed to evaluate the efficacy of acupuncture for acute migraine attacks. This study will be conducted in the Second Affiliated Hospital of Yunnan University of Chinese Medicine. Eighty patients with migraine will be recruited. The duration of the study is 10 min, including at baseline, 0–2 min (during treatment), 4 min (during treatment), 6 min (during treatment), 8 min (during treatment) and 10 min (at the end of treatment). Each group will receive 10 min of treatment, a total of one treatment. The flow chart of the study procedure is shown in figure 1. Patient enrolment, intervention and assessment schedules are shown in table 1.
Flowchart of the research procedure.
Study schedule for data measurements
Recruitment and informed consent
All participants will be recruited from the Second Affiliated Hospital of Yunnan University of Chinese Medicine and Kunming communities. A public recruitment advertisement for this trial will be designed to recruit the patients online or offline (eg, WeChat public account, websites). Neurologists will determine whether patients are eligible to participate in this study based strictly on inclusion and exclusion criteria. If patients who are interested and volunteer to participate in this trial meet the inclusion criteria, they will sign written informed consent before the start of the study (online supplemental file 2). They will be fully informed of the research information including the study procedures, benefits and potential risks, except for the information about the needling site. They are free to withdraw from the study without penalty at any time and without any negative impact on their future medical treatment.
Supplemental material
Study population
Inclusion criteria
Participants who meet all of the following requirements will be considered for inclusion: (1) meet the diagnostic criteria for MwoA in the International Classification of Headache Disorders, ICHD-3 designated by the International Headache Society in 2018; (2) unilateral migraine, male or female, aged between 18 and 60 years; (3) patients who are experiencing acute migraine attacks; (4) duration of acute migraine attacks ≤24 hours; (5) the severity of headache is moderate-to-severe (VAS 4–9); (6) volunteer to participate and sign the informed consent.
Excluded criteria
Patients will be excluded if they have (1) patients with bilateral or alternating unilateral migraine; (2) any history of head trauma, headache of other types or unknown diagnosis or cervical headache; (3) complicated with cardiovascular and cerebrovascular, liver, kidney, haematopoietic system and other serious primary diseases and other organic diseases; (4) severe anxiety, depression, insomnia and other mental diseases or intellectual disabilities, unable to cooperate with the questionnaire or infection, bleeding disorders, allergies, skin diseases; (5) patients who have already taken analgesics since the current migraine attack; (6) pregnant or lactating patients; (7) participating in other similar studies.
Removed criteria
All cases that do not meet the inclusion criteria and are mistakenly included should be eliminated; (2) patients whose trials are terminated due to serious adverse events (AEs) or complications that made it inappropriate to continue treatment.
Randomisation and blinding
The participants will be randomised in a ratio of 1:1 to the acupuncture group, and the sham acupuncture group. To avoid selection bias, random numbers were generated using SPSS V.22.0 statistical software (SPSS, Chicago, Illinois, USA) and sealed in opaque envelopes. To avoid selection bias, random numbers will be generated by an independent research assistant using a computer and sealed in opaque envelopes. After participants accept the principle of random allocation, they will randomly conduct to select an opaque envelope and obtain an allocation sequence number, which will be recorded into a case report form (CRF) by a specially assigned person. The participants, researchers, outcome assessors and statisticians will be blinded to the group allocation throughout the study. Because of the particularity of the sham acupuncture technique, acupuncturists could not be blinded.
Intervention
The intervention measures will be in accordance with the Uniform Standard for Trial Reporting16 and the Standard for Reporting Interventions in Clinical Trials of Acupuncture and Moxibustion.17 According to clinical experience, previous research and Traditional Chinese Medicine (TCM) theory (most migraine is Shaoyang meridian headache), shu points and specific acupoints of Shaoyang meridian will be selected as prescriptions. Among them, TE3 (Zhongzhu), TE5 (Waiguan), GB41 (Zulinqi), GB34 (Yanglingquan), GB20 (Fengchi) and GB8 (Shuaigu). All acupoints will be located according to the 2010 WHO standard (ISBN: Tel: 9787117123327). The acupoint locations are shown in table 2 and figure 2.
Location of acupoints
Location of acupoints.
Appliance selection
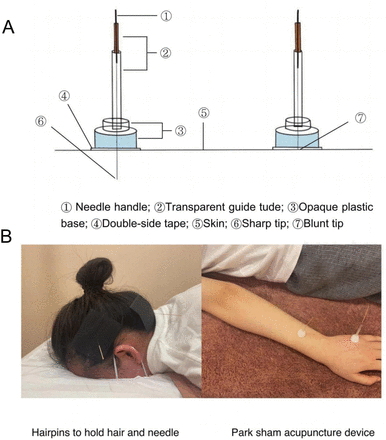
Park Sham Acupuncture Device (PSD) (see figure 3): includes transparent guide tube (Φ4×20 mm, Φ×35 mm), double-sided tape (Φ1×15 mm) and the opaque plastic base (Φ4×15 mm, Φ5×10 mm), Suzhou Medical Supplies Factory, lot number: 9018390000, China.
Acupuncture needle: use the Huatuo brand disposable acupuncture needles produced by Suzhou Medical Supplies Factory, China. Manufacturer’s license number: Su Food and Drug Administration of Machinery Production 20010020; registration certificate number: 201622770970. Specifications of acupuncture are 0.30× (25 mm, 40 mm).
Blunt needle: use retractable stainless steel blunt needle (0.25×25 mm, 0.25×40 mm), Suzhou Medical Supplies Factory, lot number: 9018390000, China.
Park Sham Acupuncture Device. Note: The person depicted is not a patient and the picture was taken with the participant’s knowledge.
Operation manipulation
Prior to the acupuncture procedure, participants will be placed in a prone position while the acupuncturist routinely sterilised the skin around the acupoints. All two groups will use the PSD.
Acupuncture group
PSD+acupuncture needle will be used. After removing the skin side of the base of the PSD double-sided tape, and then import the disposable acupuncture needle, exposing the tip of the needle, it will be fixed on the acupuncture point and pierced into the skin and the twisting, lifting and inserting, flat complementary and flat diarrhoea techniques will be performed to achieve the needling sensation occurs.
Sham acupuncture group
PSD+blunt needle will be used. After removing the skin-facing double-sided tape on the base, a retractable blunt needle will be introduced, and the blunt needle tip will retract back into the hollow needle handle by force when it touches the skin and will not pierce the skin, but the participants will feel the sensation of needle prick. The acupoints on the extremities will be glued to the skin by means of double-sided tape on the base, and on the head, which is affected by the hair, we will use hairpins to fix them (online supplemental figure 1).
Supplemental material
The duration of needle retention for both groups will be 10 min per session. Following the treatment, patients will be advised to take analgesic medication or seek other forms of pain relief if their headache persists, based on their condition. The treatment will be performed by a single acupuncturist with more than 3 years of clinical experience. Data will be collected at baseline time and the end of treatment (after 10 min) (online supplemental file 3). The table will be diligently recorded in real-time by an unbiased medical assistant during the acupuncture procedure, ensuring that they remain uninformed about the subsequent assessment of results. This approach guarantees the integrity of the data collection process and maintains impartiality during the evaluation phase.
Supplemental material
Emergency treatment
During treatment, if the patient’s headache remains unrelieved or even worsens during treatment to the extent that it is unbearable, we will discontinue the trial immediately and initiate emergency treatment. This can include emergency analgesics such as non-steroidal anti-inflammatory drugs (NSAIDs) and transporting the patient to the emergency department (figure 1) as advised by the emergency physician for further management. Additionally, if patients in the sham acupuncture group do not experience relief, they will receive acupuncture treatment or other emergency treatment.
Outcomes
Primary outcome
The primary outcome is the pain VAS score assessed before and after treatment at 10 min.18
Secondary outcomes
The secondary outcomes include the pain VAS score assessed at 0–2, 4, 6 and 8 min, blinding assessment and treatment effectiveness expectations scale.
The VAS will be assessed seven times in total, including at baseline, 0–2 min (during treatment), 4 min (during treatment), 6 min (during treatment), 8 min (during treatment) and 10 min (at the end of treatment). The VAS is an assessment tool in which patients indicate their level of pain on a continuous line of 10 cm, ranging from no pain to the worst possible pain; higher scores on the VAS indicate a more severe pain experience. The scale of the VAS of pain is shown in online supplemental file 3.
Blinding assessment will be used to assess the validity of the blinding. At the end of each session, participants will be asked to complete a questionnaire stating whether they felt they received active treatment (‘yes’ or ‘no’) and their level of confidence in receiving active treatment on a scale of 0–10. This simple and straightforward deblinded questionnaire will be made available to all participants (online supplemental file 4).
The treatment effectiveness expectations scale will be tested to gauge participants’ expectations regarding the effects of acupuncture treatment. On completing each session, participants will be asked to provide their anticipated treatment outcome (‘effective’ or ‘ineffective’) and to rate the degree to which they perceived receiving positive treatment and the expected treatment outcome using a numerical rating scale ranging from 0 to 10 (online supplemental file 4).
Supplemental material
Sample size
This study is a superiority trial designed to test whether the efficacy of acupuncture is superior to the efficacy of sham acupuncture. Based on our previous clinical study,14 the change in VAS before and after the treatment of moderately severe acute migraine with acupuncture is 5.04±1.63. We hypothesised that after 10 min of treatment. The difference in the acupuncture group is 5.04±1.63, and in the sham acupuncture group is 1.51±1.63, to evaluate whether the needling group is superior to the sham group, set α=0.025 (unilateral), β=0.1, Δ=2.3 and K=1. Using the following formula,19 we calculate the required sample size for both groups:
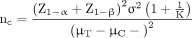
According to the formula, it is estimated that a minimum of 72 patients would be required, taking into account a loss rate of 8%. It is calculated that 40 patients should be included in each group, for a total of 80 patients.
Statistical analysis
The statistical analyses will be performed using SPSS V.22.0 statistical software (SPSS, Chicago, Illinois, USA). The detailed statistical protocol of this trial will be made by an independent statistician. Qualitative data will be described by percentages or proportions, and quantitative data will be expressed as mean±SD with a 95% CI. Independent t-test or Mann-Whitney U test and χ² test or Fisher’s exact test will be applied for the analysis of continuous and categorical variables, respectively. To assess baseline characteristics and the documentation of primary and secondary outcomes, the intention-to-treat principle will be applied. The significance level will be set at 0.05 using a two-sided test.
Patient and public involvement
Patients and the public will not be involved in the planning or design of the study. They will not be involved in the recruitment, conduct or report of the study. The study results will be disseminated to the participants and public in the forms of educational talks and booklets or flyers and published in open access peer-reviewed journals.
Trial status
The trial will begin recruitment and treatment on 1 June 2023, and is expected to be completed by 30 June 2025.
Data management and confidentiality
The researcher will fill in the initial data in the CRF of each participant in a timely and careful manner and enter them into an Excel sheet, the participants will be entered as random numbers, the researcher will not be able to identify the real information of the participants, and the data collector will be responsible for storing and managing the various types of data and the data will be strictly proofread. Each participant’s CRF will be completed in a timely and careful manner by the investigator. The data collector is responsible for keeping and managing various data and rigorously proofreading the data, and the research director will regularly check the authenticity of the data collection. Personal information of all participants, such as name, phone number, medical records, etc will be kept anonymously to prevent information leakage. The data of all participants will be kept by the researchers in a special cabinet and will be kept for at least 5 years after publication. The Ethics Committee of the Second Affiliated Hospital of Yunnan University of Chinese Medicine will regularly review the progress of the trial and supervise the collection, allocation and concealment of data. Modifications or termination of the trial could be performed by the committee. The data monitoring committee is independent of the sponsors and has no conflicts of interest.
Adverse events reporting and safety monitoring
During the study period, participants may suffer from AE, such as dizzy with needles and headache aggravated rather than alleviated, which will be given timely and proper treatment, carefully recorded in the CRF and reported to the study leader and the Ethics Committee of the Second Affiliated Hospital of Yunnan University of Traditional Chinese Medicine. All AEs will be compared between groups using the χ2 test or Fisher’s exact test.
Ethics and dissemination
This study was approved by the Ethics Committee of the Second Affiliated Hospital of Yunnan University of Chinese Medicine on 6 October 2022 (2022–008), and conformed to the Declaration of Helsinki. All participants must provide written informed consent before participating in the trial. Data handling will be done anonymously, and only the participant code available in the central database. All personal information on potential and enrolled participants out of the scope of this trial will not be collected, shared or maintained to protect confidentiality before, during and after the trial. The results of this study will be published in a peer-reviewed journal and presented at conferences.
Discussion
Migraine is a chronic disease that is difficult to cure completely and requires long-term maintenance treatment. Patients in the acute phase of the attack desperately need immediate pain relief. Studies have shown that acupuncture can inhibit sympathetic nerve function in patients with migraine, reduce the number and frequency of headache attacks and also show obvious advantages in rapid analgesia.20 Therefore, the results of this study may provide credible clinical evidence for the effectiveness of acupuncture in the treatment of acute migraine.
Migraine is often unilateral and classified as Shaoyang headaches in traditional Chinese medicine. Therefore, acupoint prescriptions usually target pain relief points situated along the Shaoyang meridian.14 In addition, as a kind of acupuncture, the action mechanism of contralateral acupuncture (CAT) is related to the central nervous system. Studies demonstrate that CAT is vital in relying on the brainstem, cerebral cortex and reticular structures within the central nervous system. By leveraging these important structural foundations, acupuncture signals are diffused and propagated bilaterally and extensively up to the brainstem level, proving beneficial in treating various pain-related illnesses.21 CAT, a classical traditional Chinese acupuncture technique, refers to the method of taking acupoints on the healthy side. In China, the CAT is widely used in pain diseases caused by various reasons and has demonstrated favourable clinical efficacy.22 23 Some studies have shown that CAT has better efficacy than ipsilateral acupuncture.24 25
In addition, migraines often occur unilaterally, with pain sites distributed to the left and right. The pathological mechanism study has found that migraine also has the phenomenon of left and right shunt of vascular distribution,26 the difference of functional response between the left and right central hemisphere27 and the asymmetry of pain-related substances (dimethylarginine, etc).28 Consequently, the inclusion criteria for this study focus primarily on unilateral migraines. The study also found that acupuncture on the healthy side could awaken the circumflex area, indicating that unilateral acupuncture can stimulate the contralateral area at the same time, providing some objective support for the treatment of painful diseases.29 Therefore, it is feasible to validate the efficacy of CAT in patients with unilateral migraine.
Some studies have shown that sham acupuncture produces greater treatment effects than drugs containing a placebo.30 In this study, we used the blunt non-penetrating needle as the control group to reduce any bias caused by opening the blind.11 31 During the acupuncture procedure, the blunt needle is touched to the skin surface as much as possible, so that the patient has a slight tingling sensation, so as to reduce the difference in needling sensation and effectively avoid the occurrence of blind bias. Previous investigations on acute pain acupuncture therapy have observed treatment effects within 15–30 min.13 32 To achieve optimal treatment effects in a shorter time frame, we based our study design on our previous research, which found that acupuncture can provide relief for migraine within 10 min.14 Thus, the observation time in this study is 10 min.
In addition, this study is the first randomised controlled study to evaluate the immediate analgesia efficacy of acupuncture for acute migraine attacks, and the findings will provide clinical evidence for acupuncture in the treatment of acute migraine attacks. Compared with medication, acupuncture offers treatment for acute migraine attacks without the potential side effects of drugs. This study can be seen in light of some limitations. First, it should be noted that this is a single-centre study. While there may be potential biases compared with a multicentre study, the quality of a single-centre study can be more easily controlled to ensure more accurate results. Second, there is no positive drug control group that was set-up in this study. Positive drugs are used as routine medicine, with controlled treatment with acupuncture and positive drugs available at a later period of time. Third, the acupuncturist was not blinded to the group allocation, which could potentially introduce some subjective bias and impact the trial’s outcomes. Furthermore, it’s worth noting that administering acupuncture treatment requires experienced and credentialed acupuncturists, which might limit its widespread popularity and practicality in clinical settings.
In summary, immediate analgesia is a major need for patients with migraine during acute attacks. Acupuncture has been considered as a better option for immediate headache relief. However, there is a lack of sufficient clinical evidence to support this. This study aims to evaluate the immediate analgesic effect of acupuncture in the treatment of acute migraine attacks, and the findings will provide reliable evidence for the application of acupuncture in acute migraine attacks.
Ethics statements
Patient consent for publication
Acknowledgments
The authors would like to thank all the patients for participating in this study and the medical workers not listed in the article.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
YH and QL contributed equally.
Contributors YH and QL contributed equally to this work and are co-first authors. TG and F-RL are co-corresponding authors. TG, YH, QL and F-RL contributed to the protocol design and writing of the manuscript. GH is responsible for the design of randomisation. XP, XT and RZ are responsible for data entry. S-WZ and ZL are responsible for acupuncture treatment. ZC and JS are responsible for guidance and statistics. All authors approved the final manuscript.
Funding This work is financially supported by the National Natural Science Foundation of China (82260964), the ‘Liang Fanrong Expert Workstation’ of Yunnan Province-Yunnan Provincial Science and Technology Plan Project (YNWR-QNBJ-2019-257), and the ‘Liu Zili Famous Doctor’ special talent programme of the Yunnan Provincial Xing Dian Talent Support Program (Yunnan Party Talent Office (2022) No. 8).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.