Article Text
Abstract
Objectives We aimed to investigate the association between repetitive proteinuria and cardiovascular events among the middle-aged and older general Japanese population.
Design Retrospective cohort study.
Setting We used repeated health screening results and medical claim data from one of the largest health insurers in Japan.
Participants Among the middle-aged and older participants (40–74 years, n=179 840), 90 752 were excluded for undergoing health screening fewer than two times and 344 were excluded for having a history of cardiovascular diseases; 88 744 who underwent kidney function screenings at least two times (from April 2011 to March 2015) were included in the analysis. Based on dipstick proteinuria test results, the participants were divided into ‘Repetitively-positive’ (positive two times or more (positive proteinuria was defined as≥1+)), ‘Once-positive’ and ‘All-negative’ groups.
Primary and secondary outcome measures The primary outcome of major cardiovascular events from baseline screening to June 2021 was hospitalisation or death due to acute myocardial infarction (AMI), cerebrovascular diseases, heart failure (HF) or peripheral vascular diseases (PVDs). The association between proteinuria and major cardiovascular events was assessed using a Cox proportional hazards model.
Results Of the 88 744 participants, 8775 (9.9%) and 5498 (6.2%) had Once-positive and Repetitively-positive proteinuria, respectively. During the follow-up period of 402 799 person-years (median 5.25 years), 660 cardiovascular events were observed, with an incidence of 1.64 per 1000 person-years (95% CI 1.52 to 1.77). Despite adjusting for major cardiovascular risk factors, we observed a high incidence of cardiovascular events in the Repetitively-positive (HR 2.08, 95% CI 1.67 to 2.59) and Once-positive groups (HR 1.36, 95% CI 1.07 to 1.72). We found similar associations for AMI, cerebrovascular disease, HF and PVD.
Conclusions Proteinuria is often repeatedly detected during annual renal screening in the general population. Repetitive proteinuria is a risk factor for major cardiovascular events.
- NEPHROLOGY
- PUBLIC HEALTH
- EPIDEMIOLOGY
- Cardiac Epidemiology
Data availability statement
No data are available. The data underlying this article are not shared due to the privacy policy of data providers.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
The study had a large number of participants and a long observation period, enabling evaluation of the risk of cardiovascular disease (CVD) in the general population.
Evaluation of urinary protein, as an indicator of exposure, could be conducted annually in the general population.
By using health check-up and insurance data, we accurately tracked the incidence of CVD outcomes.
The semi-quantitative evaluation of urinary protein was a limitation of this study.
Due to the retrospective study design, the potential impact of residual confounding factors such as the duration of diabetes and the presence of glomerulonephritis cannot be ruled out.
Introduction
The prevalence of cardiovascular disease (CVD) remains as high as 9.3% in the USA, and increased prevalence of CVD remains a worldwide health concern.1 A similar trend has been observed in Japan, where heart disease and CVD are the leading causes of death.2 It is estimated that approximately 135 000 CVD events were prevented in the USA between 2011 and 2016, while the number of CVD events has increased by approximately 170 000 in individuals aged 45–64 years.3 Screening of the risk of CVD among the middle-aged-to-older population is an important strategy in preventing CVD events.
Proteinuria is a well-established risk factor of CVD.4–12 Proteinuria is semi-quantitatively detectable by a low-cost dipstick urine test, which is performed annually and nationwide in Japan.13 According to the Kidney Disease Improving Global Outcomes guidelines, screening for urine protein is recommended only for high-risk populations, and typically, a single urine test is performed from a cost-effectiveness standpoint.14 15 A single measurement of proteinuria has been used in most general health screening programmes and epidemiological studies.5–12 16–18 Hence, the clinical significance of repetitive proteinuria has not been thoroughly discussed. There is a population that undergoes multiple proteinuria screenings and has repetitive positive results; however, the difference in the risk of CVD between single and multiple proteinuria is unclear. Therefore, the clinical significance of the second and subsequent proteinuria screenings also remains unclear.
Until recently, therapeutic interventions for proteinuria were mainly limited to renin-angiotensin-aldosterone system inhibitors. However, in 2020, treatment with sodium-glucose co-transporter 2 (SGLT2) inhibitors was shown to be effective for both heart and kidney diseases, even in the non-diabetic population, and this intervention was added to renin-angiotensin-aldosterone system inhibitors.19 20 The advent of SGLT2 inhibitors has increased the importance of proteinuria screening in preventing CVD and chronic kidney disease.
Dipstick urine tests have been performed annually and mandatorily in Japan for the general population aged 40–74 years. These data presented a unique opportunity to assess the association between annual renal screening and the risk of CVD using these data. Therefore, this study aimed to investigate the association between repetitive proteinuria and cardiovascular events using health screening data and medical claims records in Japan. The findings from this study can fill an important knowledge gap in terms of effective renal function screening in risk management for CVD in the general population.
Materials and methods
Setting and participants
In this retrospective cohort study, we obtained health screening results and medical claims data from one of the largest health insurers in Japan (a national sample of employees of civil engineering and construction companies). Using these databases, we analysed the cardiovascular outcomes after renal function screening in the general population. In the Japanese universal health insurance system, all medical care details (diagnosis, procedures and other clinical practices) are recorded in the medical claims data. Furthermore, all individuals aged >40 years are obligated to undergo annual health screening. The corresponding author had full access to all data and was responsible for data analysis.
We included participants aged 40–74 years from a nationwide health screening cohort in Japan; these participants underwent renal function screening at least two times during the baseline period from April 2011 to March 2015 (online supplemental figure S1). We excluded participants who underwent dialysis at the baseline and those who experienced any major adverse cardiovascular event (MACE) outcomes (primary endpoint) within 6 months before the baseline screening.
Supplemental material
Main exposures and covariates
Positive proteinuria was defined as proteinuria ≥1+ using a urine dipstick test. We categorised the main exposures based on at least two proteinuria results and divided the patients into the following groups: ‘Repetitively-positive’ (positive two times or more), ‘Once-positive’ (positive only once) and ‘All-negative’ groups. The covariates included age (continuous); sex (binary); estimated glomerular filtration rate (eGFR) (continuous); body mass index (BMI) (continuous); systolic blood pressure (SBP) (continuous); haemoglobin A1c (HbA1c) (continuous); low-density lipoprotein (LDL) cholesterol (continuous); history of stroke (yes, no); history of myocardial infarction (yes, no); use of antihypertensive (yes, no), antidiabetic (yes, no), or antihyperlipidaemic (yes, no) drugs; smoking (yes, no); and alcohol intake (none, <20 g/day, ≥20 g/day).
Primary outcomes
The primary endpoint was the first MACE; a composite of acute myocardial infarction (AMI), stroke, heart failure (HF) or peripheral vascular disease (PVD). We defined MACE based on the records of hospitalisations or deaths with relevant diagnosis codes defined based on the International Classification of Diseases, 10th revision (AMI: I20–25, stroke: I60–69, HF: I50, PVD: I70).21–23 The follow-up period started from the baseline screening and ended in June 2021 (online supplemental figure S1).
Statistical analyses
Baseline characteristics are presented as means with SD for continuous variables and as percentages for categorical variables, according to the proteinuria exposure groups.
Survival analysis was conducted to determine the associations between proteinuria groups and composite cardiovascular events using Cox regression models adjusted for confounders. We developed three models based on the adjusting factors: model 1—adjusted for age, sex and eGFR; model 2—adjusted for HbA1c, BMI, SBP, LDL cholesterol, antihypertensive drugs, antidiabetic drugs, antihyperlipidaemic drugs, history of myocardial infarction and history of stroke, in addition to model 1; and model 3—adjusted for smoking and alcohol intake, in addition to model 2. These covariates were selected based on clinical knowledge and previous studies.4–9 24
Secondary analysis
Subgroup and three sensitivity analyses were performed. Subgroup analyses were performed according to four cardiovascular risks: hypertension (yes/no), diabetes mellitus (yes/no), hyperlipidaemia (yes/no) and smoking (yes/no). We further analysed a subgroup without any risk factor for CVD. As these cardiovascular factors had missing values, we conducted complete case analyses to ensure that the same population was analysed in the four subgroup analyses. For the first sensitivity analysis, we categorised proteinuria 1+ and proteinuria ≥2+ and evaluated the association with MACE by excluding the effect of proteinuria ≥2+ on MACE, as participants with repetitive proteinuria were more likely to have proteinuria ≥2+. The redefined categories were as follows: at least one positive result of ≥2+ (2+ positive group), at least two positive results of 1+ with no results ≥2+ (repetitive 1+ positive group), one positive result of 1+ (once 1+ positive group) and all negative results (All-negative group). For the second sensitivity analysis, a multiple imputation approach with chained equations (‘mi impute’ command in Stata) was used to account for missing data. In total, 20 imputation datasets were used. In the third sensitivity analysis, we restricted participants to those who had undergone three or more urine tests, to minimise the impact of the number of urine tests on the results.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
Of the 179 840 participants who underwent health screenings in the database, 90 752 (50.5%) were excluded for undergoing health screening fewer than two times, 344 (0.2%) were excluded for having a history of CVDs, and finally, 88 744 who underwent kidney function screenings at least two times were analysed (figure 1). No dialysis patient had undergone this screening. Of the 88 744 eligible participants, 8775 (9.9%) and 5498 (6.2%) were in the Once-positive and Repetitively-positive groups, respectively. Detailed demographics and characteristics of the participants are presented in table 1. To summarise these characteristics, the average age was 51.6±7.9 years, and 25 341 (28.6%) patients were females. Overall, 78 453 (95.7%) patients had negative or trace baseline proteinuria, while 3726 (4.3%) patients had baseline proteinuria ≥1+. The prevalence of hypertension and dyslipidaemia was 16.1% and 9.7%, respectively. Additionally, the proportion of current smokers was 27.8%, similar to the prevalence of overall smoking reporting in Japan.25 Compared with participants in the All-negative and Once-positive groups, those in the Repetitively-positive group were more likely to have common risk factors for CVD, such as being male, high BMI, low eGFR, high blood pressure, high HbA1c level, high smoking prevalence and a history of CVD (table 1). The distribution of proteinuria severity in the excluded participants was similar to that in the included participants (online supplemental table S1).
Demographic and clinical characteristics of the participants
Inclusion and exclusion processes. CVD, cardiovascular disease.
During the follow-up period of 402 799 person-years (median 5.25 years, IQR 3.92–5.67 years), 660 MACEs were observed, with an incidence of 1.64 (95% CI 1.52 to 1.77) per 1000 person-years. Overall, 224 AMIs, 364 cerebrovascular diseases, 276 HFs and 276 PVDs were recorded during the observation period. A total of 24 522 participants dropped out due to change in the insurance system before the end of the study period. The drop-out participants were older and likely had comorbidities (online supplemental table S2).
In model 1 (table 2), the HR was 1.51 (95% CI 1.20 to 1.90) for the Once-positive group and 2.72 (95% CI 2.22 to 3.35) for the Repetitively-positive group. In model 2, which was additionally adjusted for comorbidities in model 1, the HR was 2.14 (95% CI 1.72 to 2.67) for the Repetitively-positive group. In model 3, which was additionally adjusted for current smoking and alcohol intake, the HR was 2.08 (95% CI 1.67 to 2.59) for the Repetitively-positive group.
HRs for MACE as a primary outcome in the survival analyses
We analysed the components of MACE as secondary outcomes. The incidence was 4.7 (95% CI 4.1 to 5.3), 7.4 (95% CI 6.6 to 8.2), 5.5 (95% CI 4.9 to 6.2) and 5.5 (95% CI 4.9 to 6.2) per 100 000 person-years for AMI, cerebrovascular disease, HF and PVD, respectively (table 3). The HR for the Once-positive and Repetitively-positive groups were as follows: 1.21 (95% CI 0.78 to 1.85) and 1.85 (95% CI 1.27 to 2.70) for AMI, 1.28 (95% CI 0.93 to 1.77) and 1.94 (95% CI 1.44 to 2.61) for cerebrovascular disease, 1.50 (95% CI 1.05 to 2.14) and 1.88 (95% CI 1.33 to 2.68) for HF, and 1.46 (95% CI 1.03 to 2.08) and 1.78 (95% CI 1.26 to 2.51) for PVD, respectively (table 3).
HRs for secondary outcomes in the survival analyses
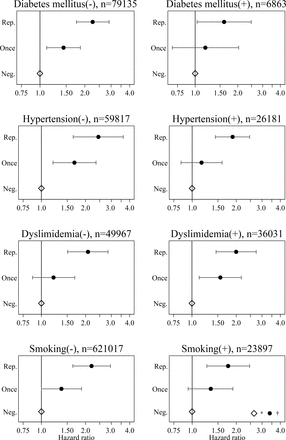
In all the subgroups, the HR was significantly higher in the Repetitively-positive group than in the All-negative (figure 2). In the subgroup without any risk factor for CVD, the HR was 2.15 (95% CI 1.15 to 4.01) and 2.81 (95% CI 1.20 to 6.56) in the Once-positive and Repetitively-positive groups, respectively (online supplemental table S3). Regardless of the cardiovascular risk factors, repetitive proteinuria was a risk factor for CVD.
Subgroup analyses by cardiovascular risk factors. *The white squares indicate the HRs in the All-negative group (reference). †The black dots with bars indicate the HRs and 95% CIs in the Once-positive and Repetitively-positive groups. Neg., All-negative group; Once, Once-positive group; Rep., Repetitively-positive group.
In the sensitivity analysis of recategorised exposures, the HR was 1.37 (95% CI 0.93 to 2.00), 1.72 (95% CI 1.05 to 2.81) and 1.89 (95% CI 1.29 to 2.77) in the Once 1+ positive, repetitively 1+ positive and repetitively 2+ positive groups, respectively. Repeated proteinuria of 1+, not including proteinuria of ≥2+, was associated with MACE. This sensitivity analysis confirmed that the frequency of proteinuria, rather than the severity of proteinuria, was a risk factor for CVDs.
We also confirmed that the effect of missing data was negligible on the result of the multiple imputation analysis. The HR in the Once-positive and Repetitively-positive groups was 1.29 (95% CI 1.03 to 1.64) and 2.01 (95% CI 1.61 to 2.50), respectively. The missing proportion was 1.42% for alcohol consumption, 2.57% for a history of CVD and 2.57% for a history of stroke, whereas the others accounted for <0.5% (online supplemental table S4). The demographics and clinical characteristics of participants with missing data are described in online supplemental table S5.
In the third sensitivity analysis, we confirmed that the results of primary analysis were independent of the definition of proteinuria and the number of urine tests. The HRs were 1.46 (95% CI 1.14 to 1.87), 2.25 (95% CI 1.59 to 3.19) and 2.12 (95% CI 1.60 to 2.82) in the single-positive, two-positive and three or more-positive groups, respectively (online supplemental table S6).
Discussion
We found that repetitive proteinuria in the screening results was associated with a high risk of MACE and its composites, including AMI, stroke, HF and PVD. To the best of our knowledge, this study is the first to show the clinical significance of repeated dipstick urine tests and report that repetitive proteinuria is a risk factor for CVDs. The dipstick urine test is a classical tool, although a more sophisticated assessment strategy can potentially make it a new cost-effective CVD risk screening tool. Dipstick urine tests are used for routine screening; thus, an important feature of the present study is that it was conducted on the general population. Similar to previous studies that evaluated proteinuria only once, we observed an association between ‘Once-positive’ proteinuria and cardiovascular outcomes.5–12 Similar to the dose–response relationship between the severity of proteinuria and the incidence of CVD, a dose–response relationship was observed between the frequency of proteinuria and CVD.7 8 10–12 Further, this relationship was observed in the subgroup analysis, and the association between repetitive proteinuria and CVD was robust regardless of the cardiovascular risk factors. Albuminuria measurements, which can detect micro-albuminuria, may be preferable to urine dipstick tests, but they are more expensive and difficult to be implemented in a mass screening programme.26 Our findings support the clinical significance of repeated urine dipstick tests to identify high-risk population for CVD in the general population.
A possible explanation for the association between CVD and proteinuria is that unfavourable CVD outcomes are mediated by the arteriosclerosis-related mechanisms of vascular endothelium dysfunction, low-grade inflammation and plaque destabilisation.3 Those mechanisms may be undeterminable by other clinical characteristics and demographics. Repetitive proteinuria may represent both the duration of underlying arteriosclerosis and a risk of CVD, which is undetermined by other clinical factors.
Two characteristics of the study database should be noted. Due to the nature of employee data, a certain number of dropouts is inevitable as retirement occurs. Since drop-out would not be associated with the presence or severity of proteinuria, it would have little impact on the outcome. Further, we analysed the data until drop-out occurred, making the study design less susceptible to drop-out. Second, the number of urine tests depended on the participants, from a minimum of two to a maximum of four. If all participants had undergone urine test screening a maximum of four times, some participants in the All-negative group would have been categorised in the Once-positive or Repetitively-positive group. This misclassification weakens the association between proteinuria and MACE, and the association observed in this study remained significant even in a conservative analysis. Thus, our results are robust for the number of urine tests at baseline.
Apart from the causal limitation due to the observational study design, this study has some other limitations. First, although the outcome was defined based on a combination of disease codes with hospitalisation and death, misclassification and upcoding derived from medical receipts may have occurred. Second, the database was mainly composed of employees or their families in a specific industry, and 71.3% of the participants were males. Therefore, we must be cautious when applying these results to the general population. Third, half of the participants in the database were excluded from the selection process. Even though the backgrounds of the excluded participants were similar to those of the included participants, a selection bias may have existed. Finally, it is important to acknowledge the presence of potential residual confounding factors, such as glomerulonephritis or infectious and autoimmune diseases.
In conclusion, proteinuria is often detected in the general population through regular renal screening with dipstick urine tests. Both single and repeated episodes of proteinuria were found to be risk factors for CVD, and a dose–response relationship was observed between the number of proteinuria episodes and the incidence of CVD. In the general population, kidney screening with repeated urine tests may help identify populations at a high risk of CVD. We need to evaluate the impact of repeated proteinuria screening, that is, whether renal screening with repeated urinalysis reduces the incidence of CVD events. These results suggest the need to redesign renal function screening strategy to address the risk of CVD in the general population.
Data availability statement
No data are available. The data underlying this article are not shared due to the privacy policy of data providers.
Ethics statements
Patient consent for publication
Ethics approval
This study was approved by the Kyoto University Institutional Review Board (IRB No. R0817) who waived the need for informed consent.
Acknowledgments
We would like to thank the insurers and their members for providing us with their health insurance data.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors TO contributed to the study design, interpretation of the data and drafting of the manuscript. YM contributed critically to the revision of the manuscript for important intellectual content. SF contributed to the study design, data analysis, data interpretation and critical revision of the manuscript for important intellectual content. SF is the guarantor who has full responsibility for the work.
Funding This work was supported by the Japan Society for the Promotion of Science (KAKENHI grant no. 19H03870 and 22H03314).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.