Article Text
Abstract
Objectives The risk factors for prematurity are multifactorial and include low omega-3 status. Omega-3 supplementation in pregnancy has been found to reduce prematurity risk, particularly among women with low omega-3 levels. This study aimed to identify maternal characteristics that predict whether women with a singleton pregnancy will benefit from omega-3 supplementation to reduce their risk of prematurity.
Design Exploratory analyses of a multicentre, double-blind randomised trial.
Setting 6 tertiary care centres in four states in Australia.
Participants 5328 singleton pregnancies in 5305 women recruited before 20 weeks of gestation.
Interventions Fish oil capsules containing 900 mg omega-3 long-chain polyunsaturated fatty acids per day versus vegetable oil capsules consumed from enrolment until 34 weeks’ gestation.
Outcome measures Early preterm birth (EPTB, <34 weeks’ gestation) and preterm birth (PTB, <37 weeks’ gestation) analysed using logistic regression models with interactions between treatment group and a range of maternal biological, clinical and demographic characteristics.
Results Omega-3 supplementation reduced the odds of EPTB for women with low total omega-3 status in early pregnancy (OR=0.30, 95% CI 0.10–0.93). No additional maternal characteristics influenced whether omega-3 supplementation reduced the odds of EPTB. For PTB, women were more likely to benefit from omega-3 supplementation if they were multiparous (OR=0.65, 95% CI 0.49–0.87) or avoided alcohol in the lead up to pregnancy (OR=0.62, 95% CI 0.45–0.86).
Conclusions Our results support previous findings that women with low total omega-3 levels in early pregnancy are most likely to benefit from taking omega-3 supplements to reduce their risk of EPTB. Understanding how other maternal characteristics influence the effectiveness of omega-3 supplementation on reducing PTB requires further investigation.
Trial registration number ACTRN12613001142729.
- NUTRITION & DIETETICS
- NEONATOLOGY
- OBSTETRICS
- PUBLIC HEALTH
Data availability statement
Data are available upon reasonable request. Deidentified data will be made available to researchers who provide a methodologically sound research proposal following review and approval by the ORIP trial steering committee and completion of a signed data access agreement. Requests can be made to maria.makrides@sahmri.com or karen.best@sahmri.com.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study uses data from the largest randomised trial of omega-3 supplementation in pregnancy with little missing data.
The maternal characteristics considered were carefully selected based on biological plausibility and clinical relevance.
The analyses were exploratory and involved many comparisons, hence significant findings may be due to chance.
The trial was designed to detect the effect of omega-3 supplementation overall, not within subgroups, hence some important predictors may not have been identified due to lack of power.
Introduction
Premature birth, especially birth before 34 weeks’ gestation, and its complications are leading causes of death and disability among children below the age of 5 years.1 Identifying effective strategies to prevent preterm birth (PTB) is therefore crucial to relieve some of the burden on infants, parents and public healthcare systems.
Omega-3 (n-3) supplementation during pregnancy is one potential strategy for preventing PTB. A recent Cochrane review of 70 randomised trials demonstrated that n-3 supplementation during pregnancy can effectively reduce both PTB (<37 weeks’ gestation) and early preterm birth (EPTB, <34 weeks’ gestation).2 In secondary analyses of singleton pregnancies from the largest trial to date, the Omega-3 to Reduce the Incidence of Prematurity (ORIP) trial,3 4 we demonstrated that the risk of EPTB fell as total n-3 status in early pregnancy increased. Further, we showed that women with low total n-3 status in early pregnancy benefited most from supplementation, achieving a 77% relative reduction in the risk of EPTB.5 Similarly, the Assessment of Docosahexaenoic Acid (DHA) on Reducing Early Preterm Birth (ADORE) trial6 reported that n-3 supplementation halved the EPTB rate among women with low DHA levels.7
While the ORIP and ADORE trials have identified various measures of maternal n-3 status in early pregnancy as predictors of whether women are likely to benefit from n-3 supplementation to reduce their risk of EPTB, there is a lack of clarity regarding whether n-3 status is the only important predictor for EPTB and whether n-3 status plays a role in the prevention of PTB. We therefore investigated whether a broad range of biological, clinical and demographic characteristics of pregnant women could be used to predict whether women will benefit from n-3 supplementation in pregnancy and hence develop a more personalised approach to supplementation to reduce the risk of EPTB and PTB.
Methods
This study used data collected in the ORIP trial, a multicentre, double-blind randomised controlled trial to assess the effect of n-3 supplementation in pregnancy on the incidence of EPTB. The trial protocol and primary trial findings have been published elsewhere.3 4 Women were eligible to participate with a single or multiple pregnancy attending one of six tertiary care centres in four states in Australia prior to 20 weeks’ gestation and the median gestational age at enrolment was 14 weeks. Women were able to participate during more than one pregnancy using a re-randomisation design8 and a total of 5544 pregnancies in 5517 women were randomised to the n-3 arm or the control arm in a 1:1 ratio. Women were provided with fish oil capsules containing approximately 900 mg n-3 long-chain polyunsaturated fatty acids (800 mg DHA and 100 mg eicosapentaenoic acid (EPA)) per day or isocaloric vegetable oil control capsules, which were designed to mimic the fatty acid profile of the Australian diet. The full compositions of the treatment and control capsules are provided in online supplemental table S1. Women were asked to consume their allocated capsules until 34 weeks’ gestation. Gestational age at delivery was determined based on the date of the last menstrual period and ultrasonographic data collected prior to 20 weeks’ gestation using a prespecified algorithm.3 The primary outcome for the trial was EPTB, while PTB was a key secondary outcome.
Supplemental material
For the present study, we restricted inclusion to singleton pregnancies from the ORIP trial with known gestational age at delivery. The outcomes of interest were EPTB (delivery before 34 weeks’ gestation) and PTB (delivery before 37 weeks’ gestation). The predictors of interest were short-listed from the detailed baseline information collected in the ORIP trial, based on biological plausibility and clinical relevance, with the same predictors selected for both EPTB and PTB. The predictors of interest included maternal age, race, socioeconomic status, education, employment, income, weight, height, smoking status, alcohol intake, use of dietary supplements, diabetes, pregnancy history and blood biomarkers of n-3 and n-6 status. The specific measures of n-3 and n-6 status considered were alpha-linolenic acid (ALA), EPA, DHA, EPA+DHA, total n-3 (sum of ALA, EPA, docosapentaenoic acid and DHA), linoleic acid and arachidonic acid in maternal capillary whole blood collected at enrolment, each expressed as a percentage of total fatty acids. DHA, EPA+DHA and total n-3 were also categorised as low, moderate or replete if levels fell below the threshold for benefit (where n-3 supplementation reduced the risk of EPTB from a relatively high level seen in the control group), between the threshold for benefit and harm, or above the threshold for harm (where n-3 supplementation increased the risk of EPTB from a relatively low level seen in the control group), respectively, as reported in previous analyses of the ORIP trial data5; no such thresholds were identified for other measures of n-3 status and hence these were not categorised as low, moderate or replete for analysis. The full list of predictors considered, their definitions (including cut-offs for defining low, moderate and replete n-3 levels and further details on how these were identified) and justifications for any exclusions from the analysis is provided in online supplemental table S2.
Patient and public involvement
Pregnant women consented to, contributed data to and were informed of the main findings of the ORIP trial according to processes described previously.3 4 There was no direct patient or public involvement in the present study.
Statistical methods
EPTB and PTB were analysed using logistic regression models. Clustering due to women who participated in the trial during more than one pregnancy was taken into account using generalised estimating equations with robust variance estimation. Due to the relatively small number of EPTBs and PTBs, each predictor was initially explored separately in a model including the randomised treatment group, the predictor and their interaction, with adjustment for the stratification variables9 (enrolment centre and use of n-3 supplements in the last 3 months). This model allows both the background risk of the outcome and the effect of treatment to vary according to the predictor. Interaction tests were performed to assess whether the effect of n-3 supplementation on the outcome varied according to each predictor (ie, effect modification) and hence whether the predictor could be used to identify women likely to benefit from n-3 supplementation. For categorical predictors, ORs are presented comparing the n-3 and control groups separately within each category of the predictor variable, along with 95% CIs. For continuous predictors, ORs and 95% CIs are presented for a 1-unit increase in the predictor variable separately by treatment group. P values from the treatment group by predictor interaction test are presented in each case. The linearity assumption was assessed for each continuous predictor using the Hosmer-Lemeshow goodness of fit test and predictors were categorised into quartiles if the model fit was questionable. Additional logistic regression analyses were performed to explore the combined effect of multiple predictors that individually identified women likely to benefit from supplementation.
Analyses were performed using Stata V.16.1 (StataCorp: College Station, Texas, USA) based on the available data. All predictors considered in the analysis had <5% missing data and the majority had <0.5% missing data (online supplemental table S2). As the analysis was intended to identify maternal characteristics that predict whether women with a singleton pregnancy will benefit from n-3 supplementation, rather than estimate the causal effects of the predictors themselves, confounders of the predictor–outcome relationship were not included in the regression models and results should not be used to infer causal effects of the predictors on prematurity. No adjustment was made for multiple comparisons due to the exploratory nature of this study and hence statistically significant findings (p<0.05) should be interpreted with caution.
Results
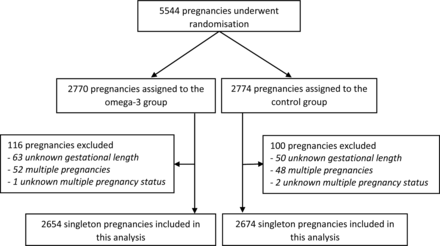
The analyses included 5328 singleton pregnancies in 5305 women with known gestational length (figure 1). Ninety-one (1.7%) pregnancies resulted in EPTB, while 378 (7.1%) pregnancies resulted in PTB. Reassuringly, baseline characteristics were comparable between the treatment groups for the subset of pregnancies included in this study (table 1), which included 96% of the 5544 pregnancies randomised in the ORIP trial.
Flow chart of participants in the Omega-3 to Reduce the Incidence of Prematurity (ORIP) trial and the data available for this exploratory analysis.
Baseline characteristics of women for singleton pregnancies included in the analysis by treatment group
EPTB (<34 weeks)
Overall, there was little evidence to suggest that n-3 supplementation is beneficial for reducing EPTB in this large subset of singleton pregnancies (1.81% vs 1.61%, OR=1.13, 95% CI 0.74–1.71; table 2), consistent with results for all randomised pregnancies in ORIP.4 However, supplementation may be beneficial for specific subgroups of women, as several blood biomarkers of n-3 status in early pregnancy were predictive of whether n-3 supplementation reduces a woman’s odds of EPTB (interaction p<0.05; table 2). The greatest benefit of supplementation was seen among women with low total n-3 status in early pregnancy, whose odds of EPTB reduced by 70% with supplementation (OR=0.30, 95% CI 0.10–0.93, interaction p=0.007). Of note, the background risk of EPTB was highest among women with low total n-3 levels; 3.05% of low status women, 1.81% of moderate status women and 0.85% of replete status women in the control group had an EPTB (table 2). N-3 supplementation had a negative effect on EPTB among women who already had replete levels of n-3 at baseline and hence had the lowest background risk of EPTB (ORs 4.50, 4.17 and 2.60 for DHA, EPA+DHA and total n-3, respectively; table 2). The possibility of using multiple biomarkers of n-3 status (DHA, EPA+DHA and total n-3) in combination to better identify women likely to benefit from n-3 supplementation was investigated but did not produce meaningful results due to the high correlation between the different status measures.
Results of interaction analyses involving blood biomarkers of omega-3 status in early pregnancy as predictors of the effect of omega-3 supplementation on EPTB
Aside from blood biomarkers of n-3 status, no additional maternal characteristics were identified as being useful for predicting whether a woman will benefit from n-3 supplementation in pregnancy to reduce her odds of EPTB (online supplemental table S3).
PTB (<37 weeks)
Consistent with our earlier report,4 n-3 supplementation may be beneficial overall for reducing PTB (6.37% vs 7.82%, OR=0.80, 95% CI 0.65–0.99; table 3). The most promising characteristics for identifying women who are more likely to benefit from n-3 supplements in pregnancy to reduce their odds of PTB were parity and alcohol use in the lead up to pregnancy (table 3, online supplemental table S4). Ignoring alcohol consumption, n-3 supplementation reduced the odds of PTB by 35% for women with a previous birth (OR=0.65, 95% CI 0.49–0.87) but did not alter the odds for first-time mothers (OR=1.03, 95% CI 0.75–1.42, interaction p=0.04). Similarly, ignoring parity, n-3 supplementation reduced the odds of PTB by 38% among women who abstained from drinking alcohol in the 3 months leading up to pregnancy (OR=0.62, 95% CI 0.45–0.86), while no effect of n-3 supplementation was seen among women who consumed alcohol leading up to pregnancy (OR=0.97, 95% CI 0.73–1.30, interaction p=0.04).
Results of interaction analyses involving alcohol use, parity and their combination as predictors of the effect of omega-3 supplementation on PTB
Further analyses suggested that the combination of parity and alcohol use may be useful for identifying women likely to benefit from n-3 supplementation to reduce their odds of PTB (three-way interaction p=0.02). N-3 supplementation was beneficial with similar estimated effects (ORs ranging from 0.57 to 0.66) for all subgroups of women defined by these two factors, except those women who were primiparous and drank alcohol in the lead up to pregnancy, among whom supplementation appeared harmful (OR=1.54, 95% CI 1.01–2.34; table 3). Other maternal characteristics varied across these subgroups defined by parity and alcohol use, highlighting that prediction does not imply causation (online supplemental table S5). Blood biomarkers were generally similar between subgroups, though the subgroup of women who may be harmed by supplementation (those who were primiparous and drank alcohol) had the highest median total n-3 levels at baseline.
Aside from parity and alcohol use, no other maternal characteristics were found to predict the effect of n-3 supplementation on PTB (interaction p>0.05), including total n-3 status (interaction p=0.23; online supplemental table S4). However, if only those women with low total n-3 levels were to take n-3 supplements (in order to reduce their risk of EPTB, based on previous recommendations5 7 and consistent with our findings above), this would be expected to reduce their odds of PTB by 44% (OR=0.56, 95% CI 0.34–0.92); limited benefits of n-3 supplementation were observed in women with moderate (OR=0.88, 95% CI 0.62–1.24) or replete (OR=0.93, 95% CI 0.66–1.30) total n-3 levels.
Discussion
In this detailed reanalysis of the ORIP trial data, we confirmed a beneficial effect of n-3 supplementation at about 1 g/day on EPTB for women with a singleton pregnancy and low total n-3 status, which is consistent with previous secondary analyses from the ORIP trial5 and findings from the ADORE7 and Chinese10 trials. We found that women with higher n-3 status in early pregnancy are already at low risk of EPTB and moderately high n-3 supplementation may increase this risk, while those with lower n-3 status are at higher risk of EPTB and are expected to benefit from higher dose supplementation. Our EPTB findings therefore support current Australian recommendations for women who are low in n-3 to take n-3 supplements during pregnancy.11 No additional maternal characteristics were found to be useful for refining these recommendations, suggesting that EPTB can be reduced in practice by measuring n-3 status in early pregnancy and recommending higher dose n-3 supplements only to those women with low total n-3 levels.12 This test-and-treat approach is currently being evaluated in South Australia by offering n-3 testing as part of an existing antenatal screening programme available without cost to all pregnant women. Such an approach could be adopted more broadly using recommended cut-points for defining low total n-3 status in whole blood (<4.2% of total fatty acids), plasma (<3.7%) or serum (<3.7%).13 Targeting supplements to women may also aid sustainability of marine sources of n-3 fatty acids.
Our exploration of PTBs among women with singleton pregnancies did not identify n-3 status in early pregnancy as a predictor of the effectiveness of n-3 supplementation, possibly due to a lack of power, although a substantial 44% reduction in the odds of PTB would be expected by supplementing women with low n-3 status in order to reduce their risk of EPTB. Interestingly, parity emerged as a potential characteristic to identify women who may benefit from taking higher dose n-3 supplements during pregnancy to reduce their risk of PTB, with supplementation beneficial for multiparous women. This is consistent with previous research showing that n-3 status is lower in women during second or subsequent pregnancies and for women with a shorter interpregnancy interval.14 The depletion of maternal n-3 stores is thought to be due to the preferential transfer of n-3 to the fetus during the third trimester.15 Parity is easy to assess and hence could be used as a simple tool, without the need for a blood test, to identify women who may benefit from n-3 supplementation. The potential value of preconception dietary strategies to rebuild maternal n-3 stores between pregnancies could also be explored, as suggested by others.16 However, our findings require confirmation in other studies before using parity to inform practice, due to the exploratory nature of this study with no correction for multiple comparisons and hence a high risk of chance findings.
Another maternal characteristic that emerged as a predictor of benefit from higher dose n-3 supplements to reduce the risk of PTB was avoiding alcohol in the 3 months leading up to pregnancy. However, if alcohol use was combined with parity, the benefits of n-3 supplementation were seen across all women except those who had not had a previous birth and who drank in the lead up to pregnancy. These complex findings are difficult to explain and may relate to inaccuracies in self-reported alcohol intake or be driven by other maternal characteristics, or combinations of them, that we either lack the power to identify or did not assess. For example, women with higher socioeconomic status are more likely to consume alcohol during pregnancy17 18 and socioeconomic status could be an important predictor of the effectiveness of n-3 supplementation.
The key strengths of this study are the large sample size (5328 pregnancies) with very little missing data, the broad range of maternal characteristics that were available for consideration and the selection of characteristics based on their biological plausibility and clinical relevance. The main limitations are the exploratory nature of the analyses involving many comparisons that may be underpowered, and the relatively few cases of EPTB and PTB, which restricted our ability to look at the effect of multiple characteristics in combination.
In conclusion, our results confirm that among women with a singleton pregnancy, those with low total n-3 levels are the ones most likely to benefit from taking n-3 supplements to reduce their risk of EPTB. Understanding how other maternal characteristics, such as parity, influence the effectiveness of n-3 supplementation on reducing PTB requires further investigation.
Data availability statement
Data are available upon reasonable request. Deidentified data will be made available to researchers who provide a methodologically sound research proposal following review and approval by the ORIP trial steering committee and completion of a signed data access agreement. Requests can be made to maria.makrides@sahmri.com or karen.best@sahmri.com.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and the Women’s and Children’s Health Network Human Research Ethics Committee (HREC) provided approval (HREC/13/WCHN/10) on 1 May 2013 for the original study and the analysis reported in the present paper. Written informed consent for the present study was obtained from all participants as part of the original study and all procedures were conducted in accordance with the approval of the relevant HREC at the six sites involved in the multicentre trial. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The authors thank Monika Skubisz, Rosalie Grivell, Philippa Middleton and Jacklyn Chen for their helpful input into the planning of this project, including the selection of maternal characteristics to consider as predictors (MM and RAG). We are grateful to the women who participated in the trial and the trial staff who collected the data used in this study.
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @SimmondsLA, @kpb2901
Contributors LNY and MM designed the study. LNY performed the statistical analyses and led the first draft of the manuscript. LNY, SKT, FH, TS, SD, ISZ and MM attended regular meetings to discuss the direction of the study and interpret the results. LAS, KPB, RAG and MM provided input into the selection of maternal characteristics to consider as predictors. All authors critically revised the manuscript and approved the final version for submission. LNY is responsible for the overall content as guarantor.
Funding Funding for these exploratory analyses of the ORIP trial data was provided by Société des Produits Nestlé S.A. under a Research Collaboration Agreement with South Australia Health and Medical Research Institute, and the National Health and Medical Research Council (APP1135155).
Competing interests RAG has received supplies from Croda UK, prepared supplies for a trial for Efamol/Wassen UK and holds a patent (WO2013/104025A1) on stabilising and analysing fatty acids in a biological sample stored on solid media, owned by Adelaide Research and Innovation, The University of Adelaide, and licensed to Xerion. SKT, FH, SD and ISZ are employees of Société des Produits Nestlé (SPN). MM has received supplies from Croda UK and prepared supplies for a trial for Efamol/Wassen UK.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.