Article Text
Abstract
Introduction Follow-up after an episode of colonic diverticulitis is a common indication for colonoscopy, even though studies have shown a low risk of positive findings in this population. Our objective is to investigate colon capsule endoscopy (CCE) as a follow-up examination in patients with colonic diverticulitis compared with colonoscopy, particularly regarding patient satisfaction and clinical performance.
Methods and analysis We will conduct a single-centre prospective randomised controlled trial. Patients seen at Odense University Hospital with acute diverticulitis confirmed by CT will be included and randomised to either follow-up by colonoscopy or CCE. Detection of suspected cancer, more than two polyps or any number of polyps larger than 9 mm in CCE will generate an invitation to a diagnostic colonoscopy for biopsies or polyp removal. We will compare colonoscopy and CCE regarding patient satisfaction and tolerance, the number of complete examinations, the number of patients referred to a subsequent colonoscopy after CCE and the prevalence of diverticula, polyps, cancers and other abnormal findings.
Ethics and dissemination Informed consent will be obtained from all participants before randomisation. The study was approved by the regional ethics committee (ref. S-20210127) and the Danish Data Protection Agency (ref. 22/43235). After completion of the trial, we plan to publish two articles in high-impact journals. One article on both primary and secondary outcomes.
Trial registration number NCT05700981.
- Endoscopy
- Clinical Trial
- Diagnostic Imaging
- Patient Reported Outcome Measures
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The single centre design.
Clinical data retrieved from patient charts can be of varying quality.
The implementation of colon capsule endoscopy (CCE) for diverticulitis follow-up within a department that is very familiar with the procedure.
This is, to our knowledge, the first study investigating CCE as an alternative modality for follow-up after acute diverticulitis.
Introduction
Colonic diverticulosis is a common condition with a prevalence that increases significantly with age.1 It has been suggested that more than two-thirds of the adult population will eventually develop colonic diverticulosis.2 The condition is often found incidentally during colonoscopy or CT, as only 20% of patients with colonic diverticulosis present with symptoms. A study found that approximately 4% of patients experienced progression from colonic diverticulosis to diverticulitis, but only 1% had the diagnosis confirmed by imaging modalities or surgery.3 Diverticulitis can, in some cases, lead to hospitalisation. If acute diverticulitis is found and verified by CT, but no radiological or clinical signs of complications are seen, patients are discharged. In patients with signs of complicated diverticulitis, hospital admission for treatment with intravenous antibiotics or surgical interventions is relevant. Several predictors for the severity of disease have been identified such as C reactive protein level and comorbidities.4 When grading the severity of disease the Hinchey classification5 based on findings during surgery and a modified Hinchey classification6 based on CT findings are often used.
In the Danish healthcare system, all patients regardless of the severity of the disease are referred for a follow-up colonoscopy 4–6 weeks after discharge to confirm the diverticulitis diagnosis and exclude coexistent malignancies. The efficiency of this approach for detecting colorectal neoplasia has been questioned in a recent meta-analysis, showing that only 6.9% of patients presented with advanced colorectal neoplasia and only 2.1% (0.5% of patients with uncomplicated diverticulitis) presented with colorectal carcinomas.7 These findings suggest that routine colonoscopy could be omitted in patients with uncomplicated diverticulitis. One alternative procedure for follow-up is colon capsule endoscopy (CCE), a procedure ideal for populations with a low risk of neoplastic findings requiring subsequent interventions, which seems to be the case for the population that are sent to follow-up after acute diverticulitis. Compared with colonoscopy, CCE is better tolerated by patients, has a very low risk of complications, and can be performed out-of-hospital.8 9 Despite these advantages, no research has been published that uses CCE as a follow-up modality in patients with colonic diverticulitis.
The objective of this study is to evaluate the effect of using CCE as a follow-up examination in patients with colonic diverticulitis compared with a colonoscopy on patient satisfaction and clinical performance.
We hypothesise that using CCE as a follow-up examination for colonic diverticulitis patients will improve patient satisfaction while reducing the need for colonoscopy, thereby benefitting patients while relieving strained colonoscopy capacities at hospitals.
Methods and analysis
Study design
Our study is a single-centre randomised, controlled trial carried out at the Surgical Department and Emergency Department at Odense University Hospital in the Region of Southern Denmark. We will use an intention-to-treat approach with two parallel arms; an intervention group (CCE) and a control group (colonoscopy) in a 1:1 allocation ratio. A structured summary of the trial information is presented in online supplemental appendix A.
Supplemental material
Inclusion and randomisation
Patients assessed without the need for hospitalisation after confirmation of diverticulitis by CT will be referred to the surgical outpatient clinic after 14 days and informed about the study by one of the researchers involved in the trial. Verbal and written informed consent will be obtained. In patients needing hospitalisation, informed consent will be obtained by involved researchers before discharge if possible. Otherwise, the patient will be referred to the surgical clinic after 14 days.
Patients who are eligible and consent to participate will be enrolled; otherwise, patients will be referred to colonoscopy according to local guidelines. When enrolled, the trial manager will conduct randomisation using a computer-generated allocation tool integrated into the software Research Electronic Data Capture (REDCap consortium, Vanderbilt, Netherlands). The randomisation sequence will not be available to any involved parties. Randomisation will be carried out for each patient individually when consent for participation is obtained.
Inclusion criteria
Patients above the age of 18 with in-hospital CT-diagnosed diverticulitis (uncomplicated diverticulitis or complicated diverticulitis modified Hinchey grade 1–3).6
Exclusion criteria
Imaging of the colonic mucosa within the last 12 months and, therefore, no indication for renewed endoscopy evaluated by the attending physician.
Colonic CT findings that require biopsy (suspected cancer) or polyp removal.
CT-verified stenosis in the gastrointestinal tract.
Cardiac pacemaker.
Renal insufficiency.
Pregnancy/breast feeding.
Allergies towards active substances administered in the trial.
Unable to provide oral and written informed consent.
Intervention arm (CCE)
Any patient allocated to the intervention arm will have a CCE using the PillCam Colon 2 (Medtronic, Minneapolis, Minnesota, USA). On inclusion and allocation to the intervention arm, patients will be contacted by a dedicated CCE nurse from an external private contractor. The CCE procedure and necessary preparation will be explained thoroughly. In case of additional questions, patients can contact the nurses by telephone during office hours (Monday to Friday, 08:00–15:00). Before CCE, participants will have to undergo bowel preparation. The bowel preparation kit will be distributed by post and is to be commenced at home, beginning 72 hours before the CCE. The kit contains polyethylene glycol (PEG) sachets (Movicol, Norgine Danmark A/S, Herlev, Denmark), PEG solutions (MoviPrep, Norgine Danmark A/S, Herlev Denmark) and instructions on how to perform the preparation properly. The details of the bowel preparation procedure are specified in table 1. The bowel preparation for CCE is more extensive than for colonoscopy due to the lack of rinsing and suctioning capabilities. For good visualisation of the mucosa in CCE it is therefore necessary to achieve a more thorough bowel cleansing than for colonoscopy.
Bowel preparation procedure
On the day of capsule ingestion, participants will take a 2 mg tablet of prucalopride (Resolor, Shire Pharmaceuticals Ireland, Dublin, Ireland) before they swallow the capsule. Afterwards, the participants will be handed the boosters (Eziclen, Ipsen Pharma, Boulogne-Billancourt, France) and will return home, where they will mix the solution with water to a volume of 1 L, of which a third is to be ingested at signals one to three, respectively. The receiver will give these signals; signal one when the capsule reaches the small bowel and signals two to four every two hours after signal one. Physical activity and the use of chewing gum are encouraged during the investigation. After signal four, a bisacodyl suppository (Dulcolax, Sanofi, Paris, France) is to be inserted. The capsule is usually excreted 4–6 hours after ingestion. After the examination is completed, the participant will return the receiver the next workday to the out-clinic facility, where all endoscopic video material will be uploaded to a secured storage function for diagnostics. We consider the CCE investigation complete if the capsule is excreted within the battery lifetime or has recorded the anal cushions in participants with acceptable bowel preparation. We define adequate bowel preparation as fair or better for all colonic segments, as evaluated using the Leighton-Rex grading scale.10
If a CCE investigation is incomplete for any reason (incomplete colon transit, bowel preparation evaluated as poor on the Leighton-Rex scale, no visualisation of the cecum, technical interruptions precluding a satisfying evaluation), the patient is referred to a colonoscopy to complete the examination.
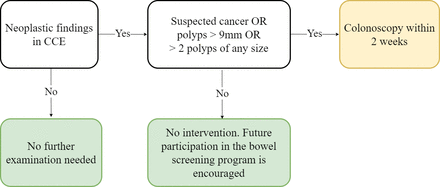
Detection of suspected cancer, more than two polyps or any number of polyps larger than 9 mm will generate an invitation to a diagnostic colonoscopy within 2 weeks for biopsies or polyp removal, and the patient will subsequently enter the standard treatment protocol. Patients, where CCE shows no neoplastic findings or what we consider low-risk polyps (defined as 1–2 polyps smaller than 10 mm), need no further investigation but are encouraged to accept future screening invitations if eligible (figure 1). According to European Society of Gastrointestinal Endoscopy guidelines, polyps larger than 5 mm detected in CCE should be referred for polypectomy.11 In diminutive polyps (<6 mm) and small polyps (6–9 mm) features of advanced histology (high-grade dysplasia or invasive cancer) are estimated to be found in 0.1%–0.3% and 0.3%–0.6% of polyps, respectively.12 In the referenced review the 5-year colorectal cancer death rate for patients with 6–9 mm polyps that are left unresected is estimated to be 0.08%. Furthermore, we have previously shown that the polyp size estimated in CCE is larger compared with that in colonoscopy.13 Considering this, we find it safe to refrain from colonoscopy in patients with only 1–2 diminutive or small polyps when encouraging future screening participation. This approach has been evaluated and approved by the regional ethics committee.
Patient flow based on colon capsule endoscopy (CCE) findings.
Control arm (colonoscopy)
Patients allocated to the control arm will undergo bowel preparation and colonoscopy in accordance with the local guidelines at the Department of Surgery, Odense University Hospital. The examination date and information are sent to the patient through their electronic mailbox.
The degree of sedation (conscious sedation using midazolam and pethidine, anaesthesiology-assisted sedation using propofol, general anaesthesia) and the specific dose of medication will be registered.
Interim analysis
Adverse events, complications and investigation quality will be monitored by the trial manager continuously during the inclusion of participants to ensure patient safety. When inclusion reaches 20 individuals for CCE investigation, the entire author team will meet and go through each adverse event registered, if any, to determine whether the trial can be safely continued. The final decision lies with the trial manager.
Objectives
Our primary objective is to compare colonoscopy and CCE regarding patient satisfaction and tolerance during the bowel preparation, during the diagnostic procedure and 4 weeks after the procedure. These patient-reported outcomes and an evaluation of patient preferences will be investigated using questionnaires.
Our secondary objectives are to compare the number of complete examinations between the intervention and control arms, to determine the number of patients referred to a subsequent colonoscopy after CCE because of identified pathology or incomplete examination and to compare the prevalence of diverticula, polyps, cancers and other abnormal findings between colonoscopy and CCE.
Questionnaires
We will ask enrolled patients to complete two to three questionnaires (Q1: online supplemental file 1, Q2: online supplemental file 2 and Q3: online supplemental files 3 and 4) longitudinally following their examination trajectory. Q1 is structured into five modules. Module 1 investigates the demographics needed for our definitions of cohort profiles. Module 2 investigates baseline characteristics and behaviour affecting health needed for our definitions of cohort profiles. Module 3 investigates bowel preparation needed for participant experience and discomfort estimations, especially since the bowel preparation regime has been intensified compared with previous methods. Module 4 investigates the expected experience of CCE needed for our estimations of the psychological effect of inviting citizens and for comparison of expected and experienced discomfort. Module 5 investigates the expected experience of colonoscopy needed for our estimations of the psychological effect of inviting citizens and for comparison of expected and experienced discomfort. Q2 consists of one module investigating behaviour during and actual experiences of CCE needed for our estimation of the effects and possible complications and adverse events that follow CCE. Q3 consists of one module investigating the actual experience of colonoscopy needed for our estimation of the effects and possible complications and adverse events that follow colonoscopy. As part of Q3 patients will be contacted by phone by one of the affiliated researchers 1 week after CCE or colonoscopy and asked which examination they would prefer if they should undergo a new endoscopic examination. Furthermore, they are asked to state the reasons for their choice.
Supplemental material
Supplemental material
Supplemental material
Supplemental material
All questionnaires have been qualitatively validated. Patients referred for colonoscopy after CCE, will receive all three questionnaires.
Data extraction and management
Data will be retrieved from patient charts and questionnaires completed by the patients. The attending physician will collect information on age and CT findings from patient charts to identify patients eligible for inclusion. When the patient has given consent to participate, the researchers involved in the project will handle further data collection from patient charts. We will collect data regarding colonoscopy findings.
Questionnaires will be distributed to patients for digital completion. Data will be entered into a REDCap database customised for this trial. All data, including CCE videos, will be stored in secure databases available only to the researchers involved in the study. To promote data entry quality, questionnaire answers will be imported directly into the database. The database will specify validation formats and intervals for data requiring manual entry. Data will be handled according to the General Data Protection Regulation (GDPR) and the Data Protection Act.
Statistical analysis
Patient-reported outcomes concerning tolerance of and satisfaction with CCE, colonoscopy and bowel preparation procedures will be estimated using visual analogue scale (VAS). VAS scores will be treated as continuous ordinal variables with severely skewed distributions. Therefore, non-parametric tests will be applied. Univariate comparisons will be performed using the Kruskal-Wallis rank sum test. Continuous ordinal regression models will be conducted to test differences in VAS scores while adjusting for possible covariates.14 15 The completion rate of investigations will be estimated as proportions, and the randomisation arms of the study will be compared using χ2 test and multivariate log-binomial regression models. Any participant’s non-adherence to bowel preparation will be adjusted for in the regression models.
The number of patients referred to a subsequent colonoscopy after CCE, the prevalence of diverticula, polyps, cancers and other abnormal findings will be estimated as proportions, and colonoscopy and CCE will be compared using χ2test and logistic regression models.
If missing data is not less than 5%, does not exceed 40%, and is not only for the dependent variables, they will be handled using multiple imputations.16 In case of multiple imputations, complete case analyses will be performed as sensitivity analyses.
Sample size
From the interim analysis of patient-reported outcomes in another randomised trial on CCE in a bowel cancer screening population (not yet published), we found a mean discomfort score of 32.2 during colonoscopy compared with 18.6 for CCE. Applying this difference to this study and with a level of statistical significance of 5% and power of 80% a minimum of 60 patients are needed in each group. Since this power calculation was performed, based on a large sample from the previous trial we have learnt that the distribution of the perceived discomfort scores tend to not follow a normal or log-normal distribution. With no suitable transformations available, we performed a simulation with 10 000 repetitions of drawing a random sample of n=60 from each group from these data. For each sample, we compared the medians of the perceived discomfort between CCE and OC. This enabled us to estimate the power for the median test to detect a difference in medians of at least 17 to be approximately 77% at the two-sided 5% level of significance.
Patient and public involvement
Before study initiation, we presented and discussed the content and phrasing of the trial protocol and participation information with a patient representative associated with our research unit. Corrections and clarifications were made according to the received feedback.
Discussion
From a patient perspective, procedure acceptability is critical when searching for ways to improve current clinical practice. Many patients feel apprehensive about colonoscopy due to the expected discomfort during both bowel preparation and the colonoscopy itself and the fear of embarrassment.17 Furthermore, colonoscopy is not without risk of complications.18 19 Adding to this, diverticulosis is associated with the experience of greater discomfort during colonoscopy.20 When considering the low risk of positive findings related to follow-up after acute diverticulitis, these patients are likely candidates for CCE. However, achieving acceptable bowel cleansing rates for CCE similar to established standards for colonoscopy is a main limitation for the wider adoption of CCE in clinical practice.21 We have in a recent study found prucalopride to significantly increase CCE completion rate and as a consequence administered a singledose of 2 mg before capsule ingestion.22
Implementing CCE in a population where the expected reinvestigation rate due to pathological findings after CCE is expected to be low can in the end benefit the healthcare system by relieving burdened endoscopy units. Enabling the hospitals to prioritise endoscopic capacity for patients in urgent need of colonoscopy and shortening the waiting lists without compromising patient management are important goals in the currently strained healthcare system.
Strengths and limitations
We acknowledge some limitations to our study, especially the single-centre design. Although patients are included in two separate locations (Odense and Svendborg), both belong within the same department. Furthermore, we know that the quality of clinical data retrieved from patient charts can vary. Colonoscopy information is needed in this study, and we depend on the clinicians to supply the relevant information. To improve reporting on colonoscopy findings, a template is available in the patient charts that will assist the endoscopist in consistent and satisfactory reporting.
A strength of our study is the implementation of CCE for diverticulitis follow-up within a department that is very familiar with the procedure after several years of using this modality. To our knowledge, this is the first study investigating CCE as an alternative modality for follow-up after acute diverticulitis.
Ethics and dissemination
This study was approved by the regional ethics committee (journal number: S-20210127) and the Danish Data Protection Agency (ref. 22/43235). Any significant modifications to the protocol will be agreed on by the researchers responsible for the trial and presented to the ethics committee for approval prior to implementation. The study will adhere to the GDPR and the Data Protection Act. The study will be conducted in accordance with the Declaration of Helsinki. After completion of the trial, we plan to publish two articles in high-impact journals. One article on both primary and secondary outcomes. Results will be published in case of positive, negative and inconclusive results. Authorships will be assigned in accordance with the Vancouver recommendations.
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors Conceptualisation: TB-M. Methodology: TB-M, UD. Writing original draft: BS-O, TB-M, UD, LK. Writing review and editing: GB, MMIT, PVA, UD. Supervision: TB-M, GB, AK. All authors have read and agreed to the published version of the manuscript.
Funding The study was funded by the Centre for Clinical Implementation of Capsule Endoscopy (CICA).
Disclaimer CICA did not have any role in the conceptualisation of this study and will not have any influence on the execution, the interpretation of the data or the decision to publish results.
Competing interests TB-M has received grants from the Region of Southern Denmark and the Centre of Clinical Implementation for Capsule Endoscopy (CICA). TB-M is member of an Advisory Board for Medtronic regarding optimising bowel preparation in colon capsule endoscopy. Anastasios Koulaouzidis is codirector and shareholder of iCERV Ltd. He has received consultancy fees and travel support from Jinshan Ltd, Diagmed Healthcare Ltd and research support (grant) from ESGE/Given Imaging Ltd and IntroMedic/SynMed, honoraria from Jinshan and Medtronic and has participated in advisory board meetings hosted by ANKON.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.