Article Text
Abstract
Objective To obtain annual incidence trends, understand clinicopathological characteristics, and forecast the future burden of colorectal cancer (CRC) in Indonesia.
Design 11-year retrospective cross-sectional study.
Setting A national referral hospital in Jakarta, Indonesia.
Participants Data from 1584 eligible cases were recorded for trends and forecasting analyses; 433 samples were analysed to determine clinicopathological differences between young (<50 years) and old (≥50 years) patients.
Methods Trend analyses were done using Joinpoint software, expressed in annual percentage change (APC), and a regression analysis was executed to generate a forecasting model. Patients’ characteristics were compared using χ2 or non-parametric tests.
Main outcomes Analysis of trends, forecasting model, and clinicopathological features between the age groups.
Results A significant increase in APC was observed among old patients (+2.38%) for CRC cases. Colon cancer increased remarkably (+9.24%) among young patients; rectal cancer trends were either stable or declining. The trend for right-sided CRC increased in the general population (+6.52%) and old patients (+6.57%), while the trend for left-sided CRC was stable. These cases are expected to be a significant health burden within the next 10 years. Patients had a mean age of 53.17±13.94, 38.1% were young, and the sex ratio was 1.21. Prominent characteristics were left-sided CRC, tumour size ≥5 cm, exophytic growth, adenocarcinoma, histologically low grade, pT3, pN0, inadequately dissected lymph nodes (LNs), LN ratio <0.05, no distant metastasis, early-stage cancer, no lymphovascular invasion, and no perineural invasion (PNI). Distinct features between young and old patients were found in the histological subtype, number of dissected LN, and PNI of the tumour.
Conclusions Epidemiological trends and forecasting analyses of CRC cases in Indonesian patients showed an enormous increase in colon cancer in young patients, a particularly concerning trend. Additionally, young patients exhibited particular clinicopathological characteristics that contributed to disease severity.
- PATHOLOGY
- ONCOLOGY
- Gastrointestinal tumours
- Colorectal surgery
- EPIDEMIOLOGY
- Gastrointestinal tumours
Data availability statement
Data may be obtained from a third party and are not publicly available. All data relevant to the study are included in the article or uploaded as online supplemental information. Raw data was obtained from a third party and are not publicly available. All additional relevant data analyses to the study have been uploaded as online supplemental information. To obtain more details data, please contact our corresponding author.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This is the first retrospective cross-sectional study of Indonesian colorectal cancer (CRC) patients with a substantial data coverage period from 2009 to 2019.
We provide trend analysis to determine changes in the annual incidence of CRC in Indonesia based on age, tumour location, and side involvement of cancer, along with a forecasting model to estimate case patterns over the next 10 years.
This epidemiological study comprehensively analysed the difference in clinicopathological characteristics of CRC in young and old patients.
Data were taken from a single centre and might not be fully representative of other centres in Indonesia. Also, being a retrospective study, this study is susceptible to record bias and data loss from medical record retention and deterioration of pathology slides.
Data that could help explain the CRC trends, such as lifestyle, diet, alcohol use, tobacco use, family history, hereditary cancer syndromes, socioeconomic characteristics, and diagnostic test frequency, were not recorded.
Introduction
Colorectal cancer (CRC) is the fourth most common cancer globally and is becoming more common in low-income and middle-income countries.1 CRC is usually diagnosed through endoscopic biopsy or polypectomy. Microscopic examination is conducted to search for invasions. In the new era of personalised medicine, the role of anatomical pathologists has been dramatically expanded. Their role is no longer limited to providing histopathologic diagnosis but also assessing staging, margins, and prognostic parameters that can only be made available by microscopic examinations such as tumour grade, lymphovascular invasion (LVI), and perineural invasion (PNI). Further research about the pathological characteristics of CRC is essential for treatment approaches and policy-making.
Recent long-term studies discovered that young people under 50 years old are more likely to get colon cancer, primarily in high-income countries.2 3 These studies’ results suggest that the clinical, histopathological, and prognostic aspects of CRC epidemiology are also expected to encounter worrying changes.4 By 2030, the incidence of colon and rectal cancer in young people, for whom routine screening is currently not recommended, is projected to increase by 28–30% and 46–124%, respectively.5 Several Asian countries, including China, Japan, India, and South Korea, have also reported a tremendous rise in the number of young patients with CRC.2 6 This phenomenon is presumably due to rapid changes in lifestyle, diet, and genetic alterations in high-risk populations, particularly young adults.2
Epidemiological studies on CRC from other parts of Asia, including Southeast Asia, are needed since CRC cases are relatively less researched and are becoming a public health threat. Furthermore, in the population younger than 50, CRC shows a rising incidence and appears to display a more aggressive phenotype with unique genetic profiles, critical differences in somatic gene mutations, and gene methylation.7 Distinct molecular carcinogeneses and genomic profiles of CRC in Indonesia drove us to present a broader view of CRC in terms of epidemiology and clinicopathological characteristics,8–10 which has not been published by any previous investigation in Indonesia. These knowledge gaps motivated us to research how CRCs have changed from 2009 to 2019 in young patients compared with their older counterparts. We also aimed to obtain annual incidence trends, understand clinicopathological characteristics, and forecast the future burden of CRC in Indonesia.
Materials and methods
Study design, data collection, and selection process
This retrospective cross-sectional study was conducted at the Dr. Cipto Mangunkusumo Hospital, Jakarta, Indonesia, to analyse CRC incidence from 2009 to 2019 using pathological archives and hospital medical records. 11–13 Data from 2020 were not included to avoid bias due to the COVID-19 pandemic, which caused a decrease in the number of patients with CRC attending the hospital. In total, 1958 patients have had a malignant tumour of the colon or rectum based on International Classification of Diseases, Tenth Revision (ICD-10) topography (C18–C20) and morphology codes (M8140/3, M8480/3, and M8490/3) with adequate biopsy or resection specimens eligible for enrolment in this study.14 For the analysis of trends, forecasting, and clinical data, 1584 patients were selected by exclusion criteria (i.e., duplication of inputted cases, change of diagnosis or metastasis), with 433 resection samples undergoing a further analysis of pathological characteristics between two age groups, as shown in figure 1.
Study flow diagram for retrospective data collection, selection process, analysis of overall included samples and subgroup analysis of complete data in the final report. CRC, colorectal cancer; ICD-10, International Classification of Diseases, Tenth Revision.
Extraction and definition of variables
The variables of age, registration year, sex, tumour site, colonic tumour location, side involvement, tumour subsites, and specimen type were extracted directly from cancer registry data. Data on tumour size, growth pattern, histological subtypes, tumour grade, pathological tumour (pT), node status (pN), adequacy of dissected lymph node (LN), lymph node metastasis (LNM), distant metastasis, staging, LVI, and PNI were retrieved from hospital medical records and pathological reports of patients who underwent surgery.
The young patient population was defined as subjects under 50 years of age, agreeing with previous studies.15 Pathological specimens of each patient were examined under the microscope by two independent pathologists who recorded the histopathology characteristics of: pathological tumour staging, histological subtypes, growth pattern, tumour grade, LVI, and PNI. We evaluated the number of dissected LNs in agreement with other studies and WHO guidelines, with a minimum of 12 LNs taken for each case.16–18 Along with LNs, we also calculated the LN ratio (LNR), defined as the number of positive LNs divided by the number of LNs examined. LNR was a significant predictor of survival in other malignancies and could be classified into subgroups according to the following cutoffs: <0.05 (LNR1), 0.05–0.20 (LNR2), 0.20–0.40 (LNR3), and 0.40–1.00 (LNR4).19
The tumour site was defined as the location where the primary tumour originated. A category of cancers known as right-sided CRC (RSCRC) originated from the caecum, ascending colon, hepatic flexure, and transverse colon. Meanwhile, left-sided CRC (LSCRC) originated from the splenic flexure, descending colon, sigmoid colon, and rectum.20 Cancer of the caecum, ascending colon or transverse colon was referred to as proximal colon cancer. The descending colon or the sigmoid colon was the sites of distal colon cancer.14 21 Tumour size was defined as the largest dimension of the three-dimensional tumour, classified into <5 cm and ≥5 cm. Metastasis (distant metastasis) was confirmed by radiography or pathological diagnostic procedure. The WHO guideline and the American Joint Committee on Cancer eighth edition were the basis for pathological staging.17 18 Tumours with a stage of pT3–T4 or a pathological staging of pTNM III–IV were considered to be in the advanced stage.17 18 Tumours were also divided into three categories based on their subtypes: adenocarcinoma not otherwise specified (NOS), mucinous adenocarcinoma, and signet-ring cell carcinoma. The tumour growth pattern was classified into exophytic, endophytic, ulcerative, and linitis plastica.22 According to a WHO categorisation based on the percentage of gland formation in the tumour mass, tumour grade was grouped as well differentiated, moderately differentiated, and poorly differentiated.17 LVI and PNI were defined as the occurrence of each parameter in at least one slide of the pathology specimen sample.23
Statistical analysis and presentation of data
A complete dataset from biopsy and resection specimens was used to extrapolate the CRC trend over 11 years, establish forecast models, and conduct a comparative analysis of the recorded variables. Missing data from the retention of the medical records and deteriorated slides were omitted. In order to address the missing data and perform a more thorough analysis of pathological characteristics, this study employed a subgroup analysis for each measured outcome with more complete data from resection-only cases (figure 1).
Data were then recorded and processed using the SPSS V.25.0 statistical software with χ2 and its alternative tests (Fisher’s exact test, Kruskal-Wallis test or Mann-Whitney test). Analysis was performed for the young and old patient populations for clinicopathological characteristics. The mean value of quantitative parameters (number of positive and dissected LNs, LNR and tumour size) was compared between two age groups with the Student’s t-test. Annual incidence rates were quantified using the Joinpoint regression package provided by the US National Cancer Institute Surveillance Research Programme and National Cancer Institute (V.4.9.1.0).24 Joinpoint regression analysis, established by Kim et al,25 is a well-known approach used to study varying trends over time with Bonferroni adjustment.26 It automatically joined separated time series of points (years) of cases on a logarithmic scale, expressed the trends as an annual percentage change (APC), and therefore, quantified the short-term increase or decrease between two successive points of change.24 25 A Monte Carlo permutation test assessed the significance of changing trends (i.e., APC).27 Joinpoint regression analysis might be employed when the temporal trend of a given quantity (e.g., proportions, rates, counts), such as incidence and mortality (e.g., referring to cancer-related scenarios), was of interest.28–30 It is valuable to generate quantitative inferences instead of qualitative ones in epidemiological studies.24 31
This presented study also performed linear and non-linear regression analyses to construct the best-fitted model to forecast the increasing trend of CRC cases in the next 10 years (2020–2029) using Minitab 19.1 (64-bit).32–38 The model trend equation to predict CRC cases can be visualised in linear [Yt = b0 + (b1 * t)], quadratic [Yt = b0+ b1 * t + (b2* t2 ], exponential [Yt = b0 + (b1t)], or S-curve (Pearl-Reed logistic) [Yt = (10a) / (b0 + b1 * b2t)] functions, with Yt being the variable, b0 being a constant, b1 and b2 being coefficients, and t as the value of the time unit. The best-fitted model is the model which has the lower values for three of these parameters: MAPE, mean absolute percent error; MAD, mean absolute deviation; and MSD, mean square deviation, or at least for two parameters, or having the lowest value for MAPE.36 39 40 The MAPE expresses accuracy as a percentage of the error. The MAD expresses accuracy in the same units as the data, which helps conceptualise the amount of error. The MSD measures the accuracy of the fitted time series. After deciding on the models, we measured the significance of their slope using the analysis of variance (ANOVA) test for curve estimation in SPSS. Statistical analyses with a p<0.05 and a 95% CI for probability were considered significant.
Patient and public involvement statement
It was not possible to involve patients or the public in our research’s design, conduction, reporting or dissemination plans. This report complied with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for observational studies (including cross-sectional studies), as stated in the Research Checklist.41
Results
Of the 1584 people diagnosed with CRC in this study, males dominated the CRC cases registered in our centre, with a sex ratio (male: female) of 1.21. Distribution based on age groups, as shown in table 1, demonstrated that the highest proportion of CRC was found in ages 51–60 years old; the mean age was 53.17±13.94 years old, with females (52.28±13.98 years old) generally being younger than males (53.90±13.89 years old), p=0.021. Looking at more specific age groupings, the number and proportion of patients’ age was: 11–20 (11; 0.7%), 21–30 (81; 5.1%), 31–40 (225; 14.2%), 41–50 (339; 21.4%), 51–60 (432; 27.3%), 61–70 (334; 21.1%), 71–80 (135; 8.5%), 81–90 (20; 1.3%), and≥91 (7; 0.4%). The mean age of the young patient population was surprisingly very young (38.82±7.46 years old). The proportion of young patients in this centre reached 38.1% (n=604) of the total incidence (n=1584). Rectal cancer incidence was higher than colon cancer (64.3% vs. 35.7%). It was roughly equal for the percentage number of proximal and distal colon cancer (49.6% vs. 50.4%). Concerning tumour side involvement, LSCRC was still higher in proportion (82.3%). Of all cases of colon cancer, the sigmoid colon is the most often affected area, accounting for 13%.
Clinicopathological characteristics of tumours in young and old patients (n=1584)
Figure 2 elucidates changes in the trend of CRC cases in Indonesian patients (denoted as an APC) over 11 years among all patients, as well as subcategorised by age groups (i.e., young and old patients), anatomical location of the tumour (i.e., colon, rectum or colon plus rectum) and side involvement of CRC (right sided vs. left sided). Using joinpoint regression analysis, a significant APC was observed among all patients, specifically in the annual incidence of colon cancer (+6.38%) and RSCRC (+6.52%). Among young patients, notable APC was only found in colon cancer (+9.24%); meanwhile, in the old patient group, a remarkable APC was noticed in CRC as a whole (+2.38%), colon cancer (+5.11%), and RSCRC (+6.57%). Trend patterns were positive for all tumour locations, except the rectum, which experienced stagnation in old patients (+0.58%) as well as dropped among the general population (–0.09%) and young patients (–0.97%) with p>0.05. More detailed data on the trend analysis of our patients with CRC have been provided in the online supplemental files 1–3.
Supplemental material
Supplemental material
Supplemental material
Trend analysis using joinpoint regression expressed by APC of CRC incidence among 1584 patients during 11 years period of study classified by tumour locations (colorectal, colon and rectum), and side involvement (RSCRC and LRSCRC) grouping in all, young, and old patients. A positive trend for 2009–2019 was observed among CRC, colon cancer, RSCRC, and LSCRC, while rectal cancer tended to stagnate and decrease in all groups. Colon plus rectum indicated a total incidence of both locations. Plotted lines indicate an APC. *Indicates that the APC significantly differs from zero at the alpha = 0.05 level using the logarithmically transformed data permutation model in joinpoint regression analysis. APC, annual percentage changes; 95%CI, 95% confidence interval; CRC, colorectal cancer; RSCRC, right-sided colorectal cancer; and LSCRC, left-sided colorectal cancer.
This study also investigated the increase of colon cancer based on their subsites (i.e., caecum, ascending colon, transverse colon, descending colon, and sigmoid colon), which is visualised in figure 3. Significantly positive APC values were observed highest in the ascending colon (+10.60%), followed by the descending colon (+10.04%), the transverse colon (+9.88%), and the sigmoid colon (+5.84%). The caecum, on the other hand, displayed a slight negative trend with a low APC value (–0.98%, p>0.05).
Tumour subsites-specific incidence rate using joinpoint regression expressed by APC of CRC incidence among 1584 patients during 2009–2019 based on anatomical subsites of tumour in the colon. A sharp increase of cases by order in value was found in ascending, descending, transverse and sigmoid colon, respectively, while a gradual decline was observed in the caecum. *Denotes a significant change in APC vs. 0 (p<0.05) using the logarithmically transformed data permutation model in joinpoint regression analysis. APC, annual percentage changes; 95%CI, 95% confidence interval; and CRC, colorectal cancer.
Additionally, as illustrated in figure 4, several forecasting models of CRC incidences were generated using the best-fitted regression analysis. This approach predicted subsequent 10-year annual incidence rates for CRC cases using a specific case equation formula. What stands out in the analysis is that the dominance of colon cancer was expected to occur in the subsequent ten years among all groups (all, young, and old patients) with a significant increase in progression slope (p<0.001, p=0.005, and p=0.001, respectively). Likewise, CRC future trends in old patients would also steeply increase following the quadratic model (p=0.043). A similar model existed in RSCRC cases, where all patients and old patients were forecasted to grow continually, with the corresponding p-value was 0.018 and 0.006. In contrast, the prediction of rectal cancer in all patients and old patients tended to be constant and dropped among young patients in the following period. Although, at a glance, the remaining forecasts appeared will be likely increasing, their slope progression was not significant (p>0.05). The precise number of predicted cases for the next 10 years (2020–2029) can be found in online supplemental files 4–6. As shown inonline supplemental file 7 and table 2, the average future burden of CRC from 2020 to 2029 compared with the current 11-year data in all, young, and old patients was ~181 vs. 144 cases/year; ~67 vs. 55 cases/year; and ~113 vs. 89 cases/year), respectively.
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Annual incidence trends, the equation for predicting cases, and the forecast number of cases in the next 10 years using the best fitted-model regression analysis (linear, quadratic, exponential growth, or S-shaped curve model) for CRC classified by tumour locations (colorectal, colon, and rectum), and side involvement (RSCRC and LSCRC) in all, young, and old patients. Projection of a positive trend for the period 2020–2029 was observed among CRC, colon cancer, and LSCRC, while rectal cancer tended to stagnate and decrease. RSCRC was forecasted to have an increased burden in all and old patients but tended to decrease in young patients. Blue connected points show actual rates, red loosely dotted connected lines indicate a best-fitted trend, and the green densely connected dotted line indicates the forecasting trend. Yt is the variable (equation for predicted cases) and t is the time unit (year) value. The significance test results for the slope of curve estimation employing the analysis of variance (ANOVA) statistical test.*Indicates a significant progression slope (p<0.05). CRC, colorectal cancer; RSCRC, right-sided colorectal cancer; LSCRC, left-sided colorectal cancer; MAPE, mean absolute percent error; MAD, mean absolute deviation; and MSD, mean square deviation.
Comparison of the mean value of clinicopathological parameters of tumour between young and old patients
As described in table 3, most tumour sizes were equal to or more than 5 cm (61%). Distant metastasis occurred in 6.9% of all cases. Most tumours were exophytic lesions (83.1%), adenocarcinoma NOS (85.2%), well differentiated (67.7%), with a pathological tumour staging of pT3 (66.6%), having inadequately dissected LNs (56.4%) and with category of LNR1 (57.5%), tumour stage IIA (34.2%), early stage (55.2%), without LVI (61.7%), and absence of PNI (88.7%). Comparing young and old patients, there were no significant differences in clinicopathological and histopathological characteristics except for histological subtypes, adequacy of LNs sampling, and PNI. Adenocarcinoma NOS was more prevalent in old patients than in their counterparts, while the mucinous variant dominated in young patients (p=0.043). Old patients were more likely to have inadequately dissected LN than young patients (p=0.004). Young patients with CRC had more PNI than old patients (p<0.001).
Pathological characteristics of tumour in young and old patients who underwent surgical resection with complete data (n=433)
The comparison of means scores between two age groups highlighted in table 2 proves two significant differences in clinicopathological parameters of the tumor. First, the mean age value between the groups of young and old patients was extremely contrasted, with more than 23 years apart (p<0.001). In addition, the average number of dissected LNs was significantly higher in the young patient group than in their older counterparts (p=0.004).
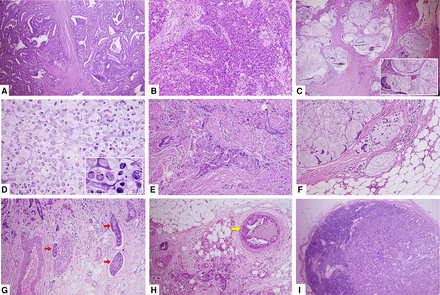
Figure 5 portrays an example of microscopic tissue images from CRC cases. This figure highlights numerous key pathological markers essential for diagnosis, identifying histological patterns, and predicting prognosis.
Histopathological features of colorectal cancer resection specimen (all in H&E staining). (A) well-differentiated adenocarcinoma NOS (×M40); (B) poorly differentiated adenocarcinoma NOS (×M40); (C) mucinous adenocarcinoma (×M40, inset ×M100); (D) signet-ring cell carcinoma (×M40, inset ×M400); (E) PT2 stage tumour infiltrating muscular layer (×M40); (F) pT3 stage tumour infiltrating adipose tissue in subserosal layer (×M40); (G) lymphovascular invasion (pointed by red arrow, ×M40); (H) perineural invasion (highlighted by yellow arrow, ×M40); (I) lymph node metastasis (×M100). NOS, not otherwise specified.
Discussion
This observational study was conducted to assess clinical trends of CRC over 11 years, forecast the future burden of CRC over the next 10 years and analyse the pathology of 1584 CRC cases in a national referral hospital in Indonesia. The current investigation corroborated previous findings regarding men’s predominance in CRC incidence. These findings are possibly due to men being more likely to smoke and drink alcohol (both risk factors for CRC), whereas women have higher levels of endogenous oestrogens, which protect against CRC carcinogenesis.42 Our study found that most CRC cases were identified in the middle-aged population, with peak incidence occurring between 51 and 60 years old, consistent with previous findings.43 Female patients had a mean age younger than male patients, consistent with findings from an investigation conducted in Brunei Darussalam.44 The definition of ‘young patients’ in an epidemiological study of CRC is arbitrary; this research employed 50 years as the cut-off age since this is the recommended age for first CRC screening in most screening programmes that have gained global adoption.15 Early-onset CRC is more likely to arise sporadically in third-world nations and is hypothesized to have a biologically and clinically unique entity, accounting for its aggressive presentation and poor prognosis.45–47 We report that CRC incidence among young patients reached nearly 40%, significantly higher than the rate reported in a previous Indonesian study on CRC between 2014 and 2016 with 275 samples (31.3%)48, Western countries (7%)49, and other Asian studies (6.7–35.5%).50 51 Other findings from South Asia were comparable to ours, with CRC incidence in young individuals ranging from 38% to 52%.46 52 The increasing proportion of young patients in our population may be influenced by the demographic profile of Indonesia, which had a high proportion of people aged 50 or lower in 2019 (79.82%).53
Trend analysis of CRC
The joinpoint regression analysis has significant analytic advantages for disease surveillance and, therefore, is valuable to portray trends in CRC incidence over time. This approach has been widely used in many CRC trend reports from several world regions, including North America (e.g., USA54 and Canada55), Latin America (e.g., Brazil56 and Mexico57), Europe (e.g., England58 and Netherland59), East Asia (e.g., China60 and South Korea61), the Middle East (e.g., Iran62 and Lebanon63), and Southeast Asia (e.g., Vietnam64 and Thailand65). Using this method, our first Indonesian study also successfully identified short-term CRC trend patterns that differed between young and old patients. CRC incidence rates were modestly elevated in all and young patients with APC+2.23% and +1.98%, respectively with p>0.05. Meanwhile, the CRC cases among subjects aged ≥50 were mounting more dramatically, with an APC of +2.38% (p=0.041). A similar conclusion was reached by Pham et al64 in the elderly Vietnamese population (APC+5.3%; 95% CI 2.8% to 7.9%). Hypothetically, this might happen because older patients were more likely to be included in screening programmes than young patients.64 Since the population-based CRC screening programme has not been implemented in routine clinical practice in Indonesia, the actual rate of early-onset CRC might have been undervalued. The estimated cost of treatment for patients with CRC in Indonesia is US$116 083.37,66 representing 0.000011% of the gross domestic product (GDP) in 2020.67 The cost burden of treatment increases significantly as the disease progresses. In terms of screening costs, colonoscopy, and faecal testing range from US$207 to US$765 and US$2.75 to US$11, respectively. Given the rising CRC incidence and high cost of treatment, but the comparatively low cost of screening, our research implies that Indonesia should adopt a population-based CRC screening programme for high-risk populations, particularly those born after 1980. This early detection will benefit the public health sector and may further reduce the economic burden.
According to WHO, the prediction of CRC incidence in Indonesia from 2020 to 2025 was higher than the APC of trend analyses done in our study (CRC: +17.7% vs. +2.23%; colon cancer: +18.1% vs. +6.38%; and rectal cancer: +17.3% vs. –0.09%).68 Further evidence showed that the trend of CRC cases among all Indonesian patients was lower in this study than in a study of patients with CRC in Tunisia from 1994 to 2009 (+2.23% vs.+3.90%).69 We also discovered a smaller APC for CRC cases among young patients compared to a study among young Thai patients between 1989 and 2012 (+1.98% vs.+5.70%).65 Trend analysis in figure 2 reveals a sharp rise in colon cancer annually among young patients with a higher APC than in old and overall patients (+9.24% vs. +5.11% and +6.38%, respectively). In the last few decades, the incidence of CRC has been increasing in Asia, particularly in Southeast Asian countries, including Indonesia and Malaysia.70 If this trend continues, the number of CRC cases may suddenly overwhelm the healthcare system. Thus, better health policies should be constructed by the government.
The rise of CRC in young patients has not yet been fully elucidated. Early life exposure to the deleterious effects of risk factors, such as frequent smoking, alcohol consumption, obesity, a Western diet, reduced physical activity, and early-life antibiotic exposure, has been thought to increase susceptibility to CRC. The first contributor is smoking, associated with hypermethylation, microsatellite instability, and BRAF mutations in CRC carcinogenesis.71 Early-life smoking may contribute to the rising incidence of CRC in young individuals.71 Concerning that fact, 13.4% of Indonesian teenagers (95% CI 12.9% to 13.9%) and 27.3% of young adults (95% CI 26.8% to 27.8%) were found to be daily smokers, respectively.72 The risk of CRC is also increased by alcohol consumption, which is positively associated with the risk of cancer of distal colon and rectum among the Asian population.73 74 In Indonesia, alcohol consumption rose strikingly from 2000 to 2020, with the current proportion of alcohol consumption in teenagers and young adults being 4.0% (95%CI 3.8% to 4.3%) and 6.4% (95%CI 6.1% to 6.6%), respectively.72 Third attributed factor should be obesity, which has been linked with a higher risk of colon cancer in Asians. In Indonesia, obese and overweight individuals comprised roughly 4–8.8% of people aged 13–18 and nearly 21% of those over 18.72 75 It is not surprising that the obesity epidemic and the rise in colon cancer happen simultaneously. Many behaviours that are thought to cause weight gain, for instance, unhealthy eating habits and sedentary lifestyles, also raise the risk of CRC. Obesity can promote cancer formation through metabolic abnormalities, hyperinsulinaemia, systemic inflammation, and alteration of the gut microbiota.76 An upward trend in CRC in Indonesia is also probably due to the acquisition of the Western diet as the fourth contributor.77 This lifestyle trend has been seen in Indonesian teenagers who consume inadequate amounts of protein, fruits, and vegetables, but excessive amounts of sodium and fast food.78 A recent study found that the de novo introduction of a Western-style high-fat, low-fibre diet induces inflammation and proliferation in the colonic mucosa within 2 weeks.79 Increasing obesity is concurrent with reductions in physical activity levels.80 A study in Japan revealed an inverse association between physical activity and CRC, and this association was stronger for colon cancer than rectal cancer.81 This fact agrees with a survey in Indonesia that found 33.5% (95% CI 33.3% to 33.8%) of the population lacked physical activity in terms of time and frequency standards.72 Another related risk factor for CRC among Indonesians is early life and improper antibiotic use.82 These two risk factors could change the gut microbiota and metabolic profile, making a person more likely to have obesity later in life.83
What stands out in the trend analysis of this study, is that the incidence rate of colon and rectal cancer was differed remarkably. In contrast with colon cancer which gradually increase (+5.11% to +9.24%), rectal cancer incidence has generally declined in all and young patients (–0.09% and –0.97%, respectively). Rectal cancer also remains stable in the old patients group (+0.58%). These findings could result from the rectum being more easily examined clinically during screening procedures than the colon, making precancerous lesions or suspected tumours easier to detect and removed during clinical examination of the rectum in screening. The negative trend of rectal cancer in this study was contradictory to the WHO’s prediction,68 but consistent with the trend in Canada.84 After 1985, rectal cancer incidence slightly declined, with an APC of –0.38% among Canadians.84 It also has been observed that the trend of CRC subsite distribution progressively shifted to the proximal colon in various countries, such as the USA (1970–2000)85, Japan (1974–1994)86, and Norway (1962–2006).87
Our findings emphasise that colon cancer incidence rose faster than rectal cancer in young patients (APC+9.24% vs. –0.97%), similar to results among Canadian young patients from 1969 to 2010 (APC+6.2% vs. +1.5%).88 The APC of colon cancer in our population was higher than in Tunisia (+6.38% vs. +4.5%).69 Some well-known risk factors do not exactly give a similar susceptibility towards colon and rectal cancer. The carcinogenic process may be different depending on where it happens.84 Diet patterns, physical inactivity, and high body mass index have been linked to a higher risk of colon cancer, but not rectal cancer.73 89 Meanwhile, smoking and alcohol consumption have been linked to a higher risk of rectal cancer.90 91 Obesity, insulin resistance, and high blood glucose levels are connected with a higher risk of colon cancer because the colon is more insulin-sensitive than the rectum.92 93 We also hypothesised that women may have benefited from the preventive effect of hormones against cancer of distal colon and rectum. Endogenous hormones may have protected some women from developing cancer of distal colon and rectum. Increased use of exogenous hormones, such as hormone replacement therapy or oral contraceptives, might also have resulted in further reductions in these cancers.42 Respecting to that evidence, 61% of Indonesian women used contraceptive management between 2005 and 2012,94 but this preventive effect has not been investigated for proximal (right-sided) colon tumours.84
In contrast to earlier findings and the widely held belief that RSCRC is more common in young patients, this study revealed that RSCRC was more frequent in old patients, similar to a study from Germany.95 Our results showed that most young patients had lesions in the left colon, in agreement with a hospital-based study in the Memorial Sloan Kettering Cancer Center, in the USA, where their young patients were more likely to have LSCRC.96 A study also claimed that LSCRC is more prevalent in men and younger individuals, whereas RSCRC is more common in women and older people.20 Accordingly, although genetic alterations might spread more in young patients and are typically related to the cause of RSCRC, it is still possible that LSCRC predominated in this group.
The trend analyses in figure 2 show that the APC of RSCRC rose statistically significantly among all patients (+6.52%) and old patients (+6.57%) over the study’s 11-year period, with the largest APC being noticed in young patients (+6.59%). The causes of these patterns remain unclear; they might be due to inconsistent plotting of several incidences each year to follow a particular joined line to figure out a trend. The rising trend of RSCRC from 2009 to 2019 could be influenced by a lack of genetic counselling addressing age, specific syndromes, and family history in Indonesia, as RSCRC is usually associated with a genetic predisposition.97 It is also challenging to detect nonpolypoid (flat or depressed) tumours, more common in the right colon. These lesions are more likely to include carcinoma but are more difficult to detect and occur more frequently in high-risk individuals.98 Higher colonoscopy miss rates may impede screening and identification of precancer and cancer lesions in the right colon, contributing to the rising trend of RSCRC.99–101 ,102 The presented study found that LSCRC had a positive steady trend among all (APC+1.41%), young (APC+1.37%), and old patients (APC+1.46%); all p-values were >0.05, similar to a report from Siegel et al103 in the USA. The clinical implications of different proportion of side involvement between young and old patients was to the aggressiveness of the disease. RSCRCs are typically bulky, exophytic, polypoid lesions projecting into the lumen and causing significant anaemia. LSCRCs are infiltrating, constricting lesions encircling the lumen, often leading to obstruction.104 A study implied that LSCRCs are genetically more unstable and phenotypically more aggressive due to distinct molecular biology patterns between RSCRC and LSCRC in DNA euploidy status, KRAS and p53 mutation rates.95
Observing more specifically the trend of colon cancer based on its subsites, what can be seen in figure 3 is the significant growth of four of five colon subsites during the study period. The APC of ascending colon rose more quickly than APC in China from 2000 to 2004 (+10.60% vs. +2.25%).91 The transverse and descending colon had opposite results (+9.88% vs. –1.95% and +10.04% vs. –1.02%, respectively), while the sigmoid colon had a more positive trend (+5.84% vs. +4.19%).105 Surprisingly, no differences in APC were found in the caecum (–0.98%), which had a slow and steady decline in cases. These trends aligned with the right-sided dominance during 11 years of study. Different parts of the colon may be more or less vulnerable to carcinogens because of biological differences in the intestine.106 For example, genetic factors may play a significant role in developing proximal colon cancer, but factors like diet, exercise, and hormone use are more likely linked to distal colon cancer.106
The trend analysis in this study enlightens us to narrow down patients in danger. Given the rapid economic transition and urbanisation occurring in Indonesia, it is possible to generalise the upward CRC incidence trend in a single centre in Jakarta to all of Indonesia,107 108 similar to what a study in Vietnam suggested.64 However, as this is a single-centre study, the data presented may not be fully representative of other centres. Further research is needed to see if the trend can be reversed, for example, by evaluating current CRC screening standards and lowering the age at which people should begin screening. To reduce the upward trend, more studies are also required to investigate CRC risk factors in Indonesia. Our current study did not record data on risk factors of CRC that might help explain the trend of CRC found in the study. Furthermore, the cross-sectional design of this study did not allow us to establish any causal relationship.
Forecasting the CRC burden
In figure 4, we forecasted the future burden of CRC by performing a fit-model regression analysis to predict colon and rectal cancer incidences along with RSCRC and LSCRC. The model with a significant slope was found in all and old patients with colon cancer and RSCRC. Meanwhile, in young patients, the model with a significant slope was found only for colon cancer. Projection models for CRC, colon cancer, and rectal cancer follow the exponential growth curve pattern in all patients and young patient group. While, in the old patient group, colon cancer and CRC forecasting models use the quadratic model. In contrast, the projection model for rectal cancer follows the linear model in the old patient group. Compared with RSCRC, which follows the quadratic model, LSCRC was more varied, with the overall occurrences following the exponential growth curve, the young patients’ incidences following the quadratic model, and the old patients’ cases following the S-shaped model (sigmoid) curve.
The best-fitted model for forecasting CRC cases had different clinical implications based on curve shape. Addressing the interpretation of each curve was challenging since little robust research explains forecasting cancer incidence.109 A linear trend is a forecasting model that develops a linear relationship between time and the response variable (incidence of disease). The linear model observed in rectal cancer among old patients means that cases increase gradually and linearly at a constant rate over time. This model assumption was based on forecast accuracy metrics and was supported by what has been pictured in trend analysis of rectal cancer with stable APC.110 What should be highlighted in this paper is that although the rectum was the most prevalent tumour site, we identified a negative trend or stable growth for this site in both joinpoint analysis and fit-model regression analysis for forecasting, similar to what was predicted in Japan.86
Six of 15 scenarios were fitted into the quadratic curve model, a forecasting method that developed a non-linear relationship between time series and the response variable. The quadratic trend resembles a polynomial regression model that accurately captures the data trend.110 Following a quadratic model, the number of RSCRC cases was expected to steeply grow after 2019, particularly among all patients (p<0.05) and old patients (p<0.01). The literature corroborates that RSCRC is associated with several adverse prognostic factors: older age, advanced stage, and mucinous histological subtype.20 111 112 On the other hand, the incidence of RSCRC among young patients was projected to plummet until 2029 (p>0.05). The future trend among young patients differs from the past between 2009 and 2019, which exhibited a steady movement. This pattern was similar to a study in the USA, which found that RSCRC increased initially, experienced stagnation, and was projected to fall by 2.3–2.6% annually.97 The reasons for different past and future trends of RSCRC might be explained hypothetically by the increased use of colonoscopy in the early 21st century.114 Along with this direction, improved techniques and training for conducting colonoscopy in the right colon to screen, detect and diagnose may contribute to reducing RSCRC lesions among subclinical diseases.113 Another possible explanation for this result is that in the previous 11-year period, our young patients were dominated by a high proportion of patients with genetic factors, thus resulting in a higher trend of RSCRC cases in the young patients’ group.8–10 In the next 10 years, the trend is predicted to shift to increasing rates of LSCRCs and RSCRCs otherwise due to greater exposure to specific cancer-related risk factors at the distal subsites.64 106 114 It is linked to the increasing adoption of a Westernised lifestyle in Indonesia, as also growing in other Asian countries, is a reasonable ground for this shift.84 115 The reasons for conflicting forecasts between young and old patients for CRC in overall and specifically RSCRC cases remain unclear. It might be explained by the complex attributions of risk factors associated with age and side of tumour involvement which has not been scrutinized in this study. Accordingly, further studies are urgently required in Indonesia to identify the contributing factors for the occurence of CRC in each subsite, thus explaining the different trends based on subsite and side involvement.
Seven cases were forecasted following the exponential growth curve as the best-fitted model. Among these forecast models, two colon cancer cases elucidated significant progression slopes; they were in all patients (p<0.001) and in young patients (p<0.01). Their future trends were identical to past trends in the previous period, even were expected to be skyrocketing. Exponential growth curve has a J-shape, reflecting a growth whose rate is proportional to the size of the population over a specific period. Exponential growth curve modelling is a regression-based method for analysing longitudinal data (i.e., tracking the same sample at different points in time), suited to the projection of trends in one disease entity into a different period. The advantage of growth curve modelling over other methods is that this technique permits the testing of several types of trajectories until the one with the best fit to the data is found, and an output is far more precise than other statistical means.116 117 Exponential growth is distinguished by its slow start and, at some point, accelerating growth rate. The exponential growth curve has the fastest growth compared with the S-shaped, quadratic, and linear curves. This pattern causes an explosion of cases, relatively more than the S-shaped, which causes a relatively constant growth rate in the population.
One scenario of LSCRC among old patients following the sigmoid (S-shaped) curve trend model refers to a case whose growth rate decreases with the increasing number of individuals.110 An S-shaped curve is symmetric around the inflection point, which means that the case increases rapidly initially, followed by a slower rate after the inflection point than the rate postulated by the curve. Following this pattern, of movement of LSCRC cases have initial slow growth, reach a growth explosion, then at their upper limit, cases will be gradually steady, consistent with insignificant slope progression (p>0.05). The S-curve trend model is best for time series that follow a logistic manner.110However, this model drawback may lead to underestimation and overestimation of the actual disease risk at the lower and upper tails of projected line.118
Projected CRC cases in Indonesia for the next 10 years confirm the future global burden of CRC, which is expected to increase by 60%, to over 2.2 million new cases in 2030.119 Looking specifically at online supplemental file 7, regarding cases predicted for 2020–2029, the burden of CRC remained high in our institution.
Distinct clinical and pathological features in young patients
Young individuals may be more susceptible to CRC due to genetic alterations and dietary changes; hence molecular profiles of young Indonesian patients with CRC have been identified to understand better the specific pathway involved in this group.8 Our young cases, mainly found in distal locations for CRC, are not in line with the characteristics of hereditary CRC, primarily found in proximal sites. They also did not follow the conventional pathways of sporadic CRC (the CIN pathway).8 Instead, carcinogenesis in these patients seems to have originated with MSI and inflammatory pathways, including cyclooxygenase-2 (COX-2) and nucleus factor κB (NF-κB). Also, lower mutation rates of the pro-oncogene KRAS are found among young Indonesian patients.8 Sudoyo et al120 found that 56.5% of CRC cases were positively stained for MSH2 and 16.5% stained for MLH1. Moreover, signet-ring cell carcinoma—an aggressive subtype of CRC that spreads rapidly and is characterised by late symptom manifestations—disproportionately affects young individuals.121 It is also possible that the differences in the immune systems of young patients could play a role in age-related immunosenescence, T-cell dysfunction, and systemic inflammation.122
Age is crucial due to its impact on prognosis. However, this idea is still debatable; some suggest worse outcomes at a young age,123 124 whereas others imply an equal prognosis between young and old age125 depending on the staging reported.43 124 Contrary to other studies,46 126 127 where stage III–IV cancer predominates in the young age group, we found that more than half of our young patients with stage I–II cancer. However, no statistically significant difference in cancer staging between the two age groups was evident, similar to a prior report.128 This might reflect increased awareness of the disease among young patients and primary care physicians, better access to colonoscopy, and more widespread use of CT with improved quality. Also, the introduction of national health insurance in the middle of the study period (2014) made access to healthcare more accessible, increasing people’s concern for their health. Providing better facilities for cancer diagnosis may result in an inflation of the number of CRC and earlier detection of CRC through screening.129 Patients with cancer found through screening show up at a much earlier stage of the disease than those not found through screening. Our study found no distinct clinical characteristics between young and old patients regarding sex, side involvement, location, site or specimen type. There is no tendency for proximalisation of colon cancer in young patients compared with old patients in our study. Overall, the proximal and distal colon had an equal proportion of all CRC cases. However, if we included rectal cancer in the calculation of distal CRC, the proportion was in line with an extensive colonoscopy survey in Asia, which found that more patients had distal than proximal CRC.130
Single institution and population-based studies have found that young patients with CRC have unique tumour locations, stages at presentation and histological features. Our findings were similar to those of these studies.131–134 The proportion of rectal cancer among young patients was significantly higher than in their old counterparts; as previously mentioned in an American study, 32% of CRC occurred in the rectum.134 Looking more specifically at colon subsites, young patients with CRC mainly have lesions originating from the ascending and descending colon. Meanwhile, the caecum, transverse colon, and sigmoid colon were the most affected sites among old populations. Lesions with poorly defined histological features, such as mucinous and signet ring features, are more likely associated with poor outcomes.123 They are also more resistant to chemotherapy.128 Our results showed that the proportion of adenocarcinoma NOS in young patients was less frequent than in old patients, agreeing with a study by Chan et al52 and Gheju et al135 . The mucinous histological variant was significantly higher in young than in old patients. Signet-ring cell cancer was only observed in young patients, accounting for only 0.6–1.0% of all CRC cases globally.135 Our single patient who has signet-ring cell cancer has the following characteristics: 48 years, female, located in the caecum, right-sided, size 5.5 cm, brown-coloured surface, exophytic, adequate LNR 5/13, pT3N2aM0 (IIIB), no LVI, no PNI, and with poor tumour differentiation. Likewise, only one patient with signet-ring cell carcinoma was also identified in a Romanian study, but that patient was elderly (>50 years).135 Signet-ring cancers have intracellular mucin pushing the nucleus to one side and are associated with a more advanced stage at diagnosis, a higher incidence of LVI, LNM, and liver metastases, a higher rate of recurrence, and higher aggression.136 137 The literature stated that mucinous histopathology was a significant predictor of poor outcomes and more advanced node stage.138
The average number of dissected LNs in our study was lower than that in a recent Romanian study (mean: 9.96±5.46 vs. median: 35.7 LNs removed), indicating that optimal LN sampling was a challenge in our institution.135 Meanwhile, the average number of positive LNs per patient was lower than positive cases in Romania (mean: 1.54±2.73 vs. median: 3.7 (1–62)).135 The interpretation of LNM is thus more complicated because the number of dissected LNs was not ideal, but the positive number was favourable, which might be masking. More insufficiently removed LNs might result in a higher probability of positive LNs in actual conditions due to unsuccessful LNs sampling, which could harm the detection of cancer spread. This issue may have an impact on patient staging. In contrast, increasing the number of dissected LNs leads to more accurate information about node status and more effective patient care. In a recent Dutch nationwide study,139 authors found that with an increasing number of evaluated nodes, the risk of mortality is decreased, related to a better quality of surgical resection (yielding more LN for the pathologist to assess).
A closer inspection of the dissected LNs in table 2 shows significant differences between the two age groups. The number of adequate LNs dissected in young patients was higher than in old patients, a favourable finding in young patients. Old patients are more likely to receive inadequate LN dissection during operative therapy, given their higher surgical risk for various postoperative complications and comorbid diseases. This concern possibly makes surgeons weigh the risks and benefits of a more thorough LN dissection.140 Other contributing factors to the number of LNs dissected from resection include the surgeon’s technique, bowel resection length, and tumour location.141 Complying with a minimum LN count of 12 is sometimes problematic, challenging, and less applicable. Thus, a novel measurement has been proposed to be used in clinical practice: LNR, a ratio of positive LNs to total dissected LNs. The mean score of LNR in our patients was was lower than a median value of LNR in a study in Romania (mean: 0.18±0.29 vs. median: 0.221 (0.139–1)).135 This value was in line with the highest proportion of lower-category LNR (LNR1 was 57.5%) in the study analysis, implying favoured results. LNR provides a superior prognostic value than the number of positive nodes alone. A higher LNR is also significantly associated with poorer survival of CRC.142 Given no statistical difference in the LNR measurement between young and old patients in this study, it might be potential for LNR to be included as a predictive indicator in CRC staging systems for all patients.
This study found a lesser proportion of PNI in all CRC patients than in Elsamany et al143 (11.3% vs. 24.4%). Nonetheless, we documented a significantly higher proportion of PNI in young patients than in old patients (p<0.001), similar to findings in Zahir et al,45 showing that 22% of their young CRC patients had positive PNI. PNI is associated with a higher rate of metastatic disease, a greater likelihood of recurrence, and poorer survival.144 Several studies have also recognised it as a notable independent prognostic factor in CRC multivariate analysis.144
Although some pathological features exhibited significant differences between the two age groups, no evidence was found for significant differences in tumour size, growth pattern, tumour grade, pT, pN, LNR, LNM, distant metastasis, and LVI. Two-thirds of patients had tumour size ≥5 cm, the most significant size being 18 cm. Although some authors believe that tumour size does not affect prognosis, others believe that tumour size partially affects prognosis.145 146 Increasing tumour size is associated with decreased loco-regional control, resulting in an increased risk of malignant potential.147 More extensive tumours are more likely to be more invasive and invade adjacent organs.148 Local recurrence was significantly higher in patients with tumours measuring ≥5 cm in size, poorly differentiated adenocarcinoma, pT4 stage, and having adjuvant radiotherapy. Moreover, the 5-year overall survival rates in patients with tumours ≥5 cm were lower than those with a size <5 cm (log-rank, p=0.001).149
According to our findings, the proportion of growth patterns (from highest to lowest, in both age groups) was exophytic, endophytic, ulcerative, and linitis plastica. These findings agree with a previous study in Thailand, which found that fungating and polyp mass (exophytic) were more common compared to ulcerative masses.149 Our research revealed that exophytic growth patterns were prevalent in all patients and were distributed equally between the two age groups. Ulcerative growth and linitis plastica were much less common, which is favourable since both growth modes entail a worse prognosis. Linitis plastica suggests de novo origin, associated with a reduced proportion of KRAS mutations. Clinically, de novo tumours may represent a more aggressive subtype of CRC with a worse prognosis, poorer disease progression, and higher aggressiveness.104 These results call for more awareness and persistence in detecting non-polypoid lesions, more intensive monitoring of colonoscopically treated cases, and surgery for selected patients.
Concerning tumour grading, most tumours in both age categories were well differentiated, similar to the results of a study from India.138 These findings differed from those of a study by Chan et al,52 who discovered that both age groups were primarily affected by cases of moderately differentiated tumours. We identified that young patients were more likely to have poorly differentiated CRC than old patients. This finding shows how young patients have predilections for more aggressive tumour biology and implies a poorer prognosis regarding distinct tumour grade and histological subtypes distribution.124 150 However, although we found notable differences in the histological subtypes of young and old patients, no evidence was found for a significant association between tumour grade and age.
LVI was detected in almost two-fifths of individuals in this study. This proportion was fewer than in a previous report on Saudi patients (49.5%).143 However, it was noticed that positive LVI cases were higher in our young patients than in old ones (41.7% vs. 36.7%). These findings suggest that LVI is a critical histopathological feature that needs to be assessed in every young patient with CRC, since literature mentioned its presence links to worse survival.143
In short, all empirical findings related to clinicopathological characteristics of CRC in this study have provided a new understanding of this disease entity in Indonesia. Our study collected CRC data archived in one of Indonesia’s national referral hospitals for cancer with a lengthy study period and is therefore the most robust data accessible in our nation. Its coverage could represent CRC epidemiology on a regional scale since primary data for the entire country is not readily available. Another strength of this study was that we applied an efficient and noteworthy statistical method called joinpoint regression analysis to study the in-depth dynamics of CRC cases in Indonesia.31 151 152 This approach has allowed estimation of the magnitude of incidences, testing the movement of cases statistically, and clearly illustrating the direction of CRC trends.24 25 153 This study also provided several best-fitted models and computed forecasts that predict future trend patterns statistically.
However, our study should be interpreted with caution in light of the following limitations related to research methodologies. As a retrospective study, the quality of our database depends on the patient records and is subjective to record bias. We also excluded patients from our study due to retention of medical records or microscopic slide deterioration. This research may also have missed some old, frail patients with symptoms of CRC who were treated at home or in nursing homes without further investigation. Furthermore, several drawbacks might also arise concerning joinpoint regression analysis to measure the trend of cases. This method’s common impediment was that it only offered a description of the time series based solely on yearly aggregated data154; thus, it could not draw a causal relationship between possible risk factors that contributed to the findings.155 As such, we could only hypothesise associations between CRC trends changes highlighted by our data and their possible influential factors supported by existing scientific evidence. Also, relying on the length of the study period, the software could only measure a certain number of year segments at a time.153 A longer research term would have offered more freedom to measure the APC in several segmented sequences.153 As a result, we could not compare several joinpoint segments to gain additional clarity regarding the impact of a specific intervention or event. The analysis could only be limited to 1 joinpoint because our samples only had 11 data points (i.e., 2009–2019).153 To exemplify, given that Indonesia initially implemented universal health coverage in 2014, this limitation might restrict the analysis to distinguish different APCs between 2009–2013 and 2014–2019.
In addition, the projections of future CRC incidence discussed in this study should be carefully interpreted.109 Predictions of future cancer incidence inherently depend on several uncertain factors, could be part of a larger cycle and may not persist into the future. Our projection of CRC in 2020–2029 was assumed to have similar clinicopathological characteristics as the circumstances observed from 2009 to 2019. Any changes affecting future cancer incidence rates beyond those included in the model’s base years could not be statistically calculated by the forecasting models.156 Dynamic evolutions in the population (e.g., advancing obesity or smoking rates and introducing new screening programmes with more cutting-edge technologies), governmental policy adjustments, and emerging public health threats (e.g., pandemics) may influence the record of a predictive number of cases.156 Trends and projections are volatile, and thus we could only forecast cases over a short period (e.g., 10 years in our study) to maintain forecasting accuracy. Moreover, this work did not include population-level data, and the mathematical prediction of cases in this study should be further validated using multicentre datasets.157 Therefore, population and multicentre epidemiological studies are highly suggested to further predict trends in this disease entity
Despite all methodology-related limitations, our data showed a similar trend to other countries worldwide, primarily Asian countries. The incidence rates fit well into forecasting models, allowing clinicians and policy-makers to predict and anticipate future disease burdens of CRC.
Conclusion
This study sets out to assess clinical trends in CRC over 11 years based on tumour locations and side involvement, forecast the future incidence of CRC for the next 10 years, and analyse the clinicopathological profile among Indonesian patients in a single centre. Epidemiological trends and forecasting of CRC cases in Indonesian patients showed an enormous increase, notably for colon cancer, with a particularly concerning trend in young patients. Forecasts for the next 10 years using fit-model regression analysis found a significantly high number of CRC burdens in the future, particularly for colon cancer compared with rectal cancer, which is stable and declining. Additionally, young patients exhibited particular clinicopathological characteristics regarding tumour location, tumour subsites, histological subtypes, adequacy of dissected LNs and PNI, contributing to the disease’s severity, aggressiveness, and prognosis. Multidisciplinary policies encompassing specialised screening protocols, extensive educational efforts, and lifestyle adjustments are required immediately to address this perplexing problem.
Data availability statement
Data may be obtained from a third party and are not publicly available. All data relevant to the study are included in the article or uploaded as online supplemental information. Raw data was obtained from a third party and are not publicly available. All additional relevant data analyses to the study have been uploaded as online supplemental information. To obtain more details data, please contact our corresponding author.
Ethics statements
Patient consent for publication
Ethics approval
Ethical approval (KET-139/UN2.F1/ETIK/PPM.00.02/2020, protocol number: 10-11-1416) was obtained from the Institutional Ethical Review Board (IERB) of the Faculty of Medicine, Universitas Indonesia. General consent for the use of medical record data and residual material had already been obtained, in line with ethical approval.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Collaborators None declared.
Contributors NR was the principal investigator of this study, conceptualised the study, acquired funding, and controlled the decision to publish. As a guarantor, NR accepted full responsibility for the overall content of the work. NR and MH did the investigation, designed the methodology, had full access to the data, contributed to the analysis, drafted the paper, and did the project administration. MH was entirely responsible for software utilisation, data cleaning, and visualisation of research findings. NR, MS, DRH, EK, MA, and WSJ collected the data, provided resources, and validated all data analyses. NR, EK, MA, and WSJ supervised the study process thoroughly. All authors critically revised the manuscript for important intellectual content, and all authors gave final approval for the version to be published.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.