Article Text
Abstract
Objectives To identify all available studies assessing the use of portable ultrasound devices for pregnant women, with the specific aim of finding evidence for devices used to determine gestational age and their validity when compared with conventional ultrasound machines. We also wanted to determine what portable ultrasound models are commercially available for obstetric use.
Design Systematic scoping review.
Primary and secondary outcome measures Extracted variables included study design, population, method of ultrasound measurement, devices used and whether studies formally validated accuracy against conventional ultrasound.
Results We searched four databases—Medline, Embase, CINAHL and Maternal and Infant Care. In total 56 studies from 34 countries were identified; most were observational studies. Across all studies, 27 different portable ultrasound models (from 17 manufacturers) were evaluated. Twenty-one studies assessed use of portable ultrasound for evaluating fetal characteristics or estimating gestational age, and 10 of these were formal validation studies. In total, six portable devices have been validated for gestational age estimation against a conventional ultrasound comparator. The web searches identified 102 portable devices (21 manufacturers). These were a mix of handheld devices that connected to a phone or computer, or laptop-style portable ultrasound devices. Prices ranged from US$1190 to US$30 000 and weight ranged from 0.9 kg to 13.0 kg.
Conclusion While the number of commercially available portable ultrasound devices continues to grow, there remains a lack of peer-reviewed, quality evidence demonstrating their accuracy and validity when compared with conventional ultrasound machines. This review identified some models that may be useful in gestational age estimation in low-resource settings, but more research is required to help implement the technology at scale.
Trial registration number Registered via Open Science Framework (DOI: 10.17605/OSF.IO/U8KXP).
- obstetrics
- ultrasonography
- prenatal diagnosis
- fetal medicine
Data availability statement
Data are available in a public, open access repository.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
We applied a detailed and tailored search strategy to a wide range of data sources to identify as many relevant studies as possible, including a variety of medical databases.
The screening and data extraction processes were completed by two independent reviewers, with any conflicts resolved by a third reviewer.
The findings from our formal scoping review were augmented by additional web searches of ultrasound manufacturers.
We acknowledge that scoping reviews do not take into account the integrity or accuracy of individual studies identified.
We acknowledge that some studies may have been published outside of the databases and websites we searched.
Background
The WHO recommends that all pregnant women should receive at least one ultrasound scan before 24 weeks’ gestation to estimate gestational age, improve detection of fetal anomalies and multiple pregnancies, reduce induction of labour for post-term pregnancy, and improve a woman’s pregnancy experience.1 An ultrasound scan for gestational age estimation is most accurate when it is performed in the first trimester of pregnancy.2 Several antenatal interventions recommended by WHO confer benefit when used at specific gestational ages—such as antenatal corticosteroids for women at risk of preterm birth prior to 34 weeks’ gestation,3 aspirin for women at increased risk of pre-eclampsia prior to 20 weeks’ gestation4 and induction of labour for post-term pregnancy5—and hence the safe and appropriate use of these interventions can be affected by accuracy of gestational age estimation. WHO’s antenatal care recommendations emphasise the need for effective and reliable antenatal ultrasound services to be available to all pregnant women, in order to optimise maternal and newborn health outcomes.6 However, in many low-/middle-income countries (LMICs), women’s access to reliable antenatal ultrasound is often limited or only available in certain contexts, such as tertiary hospitals or in private health services.7 8 Resource constraints and limited infrastructure in rural health facilities further impact the ability to implement traditional or conventional ultrasound machines in these settings.
Recent years have seen the development of portable, wireless, compact or mobile-based ultrasound systems for obstetric use.9 If such portable ultrasound devices are as accurate as conventional, cart-based ultrasound systems—as well as being easy to use, affordable and acceptable to women and their healthcare providers—they could help improve pregnant women’s access to antenatal ultrasound, and thus increase coverage. A 2016 systematic review explored available research on the use of portable ultrasound devices in the triage, diagnosis and management of adult patients in LMICs, and found 36 studies describing their use in cardiac screening, abdominal assessment, obstetric dating, and in rapid triage in rural areas or emergency settings.9 While that review identified only three studies related to portable ultrasound use in pregnancy, a number of new portable ultrasound models have become commercially available since that review was conducted, including several models intended specifically for pregnant women.
We therefore aimed to conduct a scoping review to identify all available studies assessing the use of portable ultrasound devices for pregnant women, as well as aiming to identify what portable ultrasound models are currently commercially available. We did this review to help identify which (if any) devices would be useful for improving access to antenatal ultrasound for women in LMICs.
Methods
Study design
Scoping reviews are a useful methodology for examining the range and nature of existing literature on a topic.10 11 They are well suited to addressing relatively broad questions, as they can create a map of the existing literature in a reproducible and transparent manner.12 Scoping reviews can provide insights into how a topic has been studied, and whether knowledge gaps exist. This scoping review was conducted in accordance with the Joanna Briggs Institute Methodology for Scoping Reviews, and is reported as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews standards.10 11 We first developed a review protocol which was registered online via the Open Science Framework website.13 As a systematic review of publicly available data, ethics approval was not required. No patients or members of the public were involved in the design or conduct of this review.
Patient and public involvement
No patient’s or members of the public were involved in the design, conduction, or dissemination of results for this paper.
Eligibility criteria
For the scoping review, eligible studies were primary research studies that used any study design, conducted in any country, setting or language, provided that the study involved the use of a portable ultrasound device (variably described as point-of-care, wireless, compact, or mobile-based ultrasound devices) in pregnant women. We also included studies that pertained to training healthcare providers in the use of portable ultrasound devices for pregnancy-related indications. Studies were included regardless of the comparator used. We searched the literature from 1 January 2000 onwards, considering that portable ultrasound devices are a relatively new technology. While the aim of the review was to identify portable ultrasound devices specifically for gestational age estimation, we decided to use eligibility criteria that captured any study assessing the use of a portable ultrasound device for any pregnancy-related indication, to ensure that no eligible devices or data were missed. This was also because some ultrasound devices might have multiple uses (such as gestational age estimation, assessing position of the placenta, or detecting fetal anomaly). Studies that related to the use of conventional ultrasound systems only (ie, cart-based ultrasound devices), or studies that assessed portable ultrasound use in clinical contexts outside of obstetric applications were not included. Conference abstracts, case reports, case series, study protocols and editorial letters were also not eligible. Systematic reviews were not considered eligible but were checked for any studies not identified through our searches.
Literature searching and assessment of eligibility
We searched four databases—Medline, Embase, CINAHL and Maternal and Infant Care—on 29 July 2021. With support from two information specialists, search strategies were constructed for each database, combining relevant synonyms and search terms for pregnancy (including terms related to foetal biometry and GA estimation) and portable ultrasound devices (online supplemental tables S1–S4). Identified citations were collated and deduplicated in Endnote,14 before uploading to Covidence for screening.15 Two reviewers independently screened and assessed titles and abstracts of all retrieved citations for potential eligibility. For potentially eligible studies, full texts were retrieved and assessed by two independent reviewers according to the review’s eligibility criteria. Disagreements during both stages were resolved either through discussion or consultation with a third author.
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Separate to the searches of these four databases, we used Google searches to identify portable ultrasound devices that were commercially available at the time of searching. These searches used structured search terms and synonyms to identify manufacturers of portable ultrasound systems (online supplemental table S5). We also searched individual websites of ultrasound manufacturers to identify what (if any) portable ultrasound systems were currently available (online supplemental table S6). Once the scoping review was completed, we updated these searches to ensure that manufacturers identified in the included studies were also included in these web searches.
Supplemental material
Supplemental material
Data collection and analysis
For the scoping review, data extraction was conducted using a customised Google Sheet, which was pretested and refined on five eligible studies. For each included study, we extracted data on: study title, author, year of publication, country and region where the study was conducted, study design, population, setting, stage of pregnancy, method of measurement (transabdominal, transvaginal, and/or transperineal), device used and what parameters were assessed. By parameters, we mean whether the study reported on accuracy, effects on health outcomes, feasibility, whether training programmes were used, and whether they compared findings to conventional ultrasound devices. The country where a study was conducted was classified into income levels using 2021 World Bank categories.16
Study designs were classified according to the Centre for Evidence-Based Medicine’s published hierarchies of evidence,17 while those studies that self-described as pilot, field or validation studies were classified as ‘other primary research design’. We also classified each study based on its main objective—for example, whether the study used portable ultrasound primarily for: gestational age estimation, confirming pregnancy, routine antenatal ultrasound scans, identifying ectopic pregnancy, identifying or monitoring placental abnormalities, congenital anomaly screening, monitoring labour progress, or emergency/trauma applications for pregnant women (online supplemental table S7). For those studies that formally validated a portable device against a conventional ultrasound system for gestational age estimation, the findings of that validation analysis were reported. As a scoping review, quality assessment of individual studies was not performed. Data were analysed descriptively. For the purposes of reporting review findings, the term “portable ultrasound” was used to mean any point-of-care, wireless, compact or mobile-based ultrasound device, as distinct from conventional (non-portable) or cart-based ultrasound devices.
Supplemental material
For the web searches to identify commercially available portable ultrasound devices, we extracted available data on country of manufacture, countries of registration, intended use and user, what training is provided or available, and the device characteristics. This included the device’s power supply, battery life, transducers, obstetric software presets, estimated lifetime, drop and waterproof standards, weight, dimensions, accessories, screen resolutions, software requirements, storage, data export options, price and warranty. In 2018, WHO published a policy brief on their antenatal care recommendations, identifying eight suggested requirements that obstetric ultrasound equipment should meet for antenatal care (box 1). We assessed all identified ultrasound systems against these eight requirements.6
Suggested equipment capacity for obstetric ultrasound (US; reproduced with permission from the WHO’s recommendations on antenatal care for a positive pregnancy experience)6
Real-time, grayscale capabilities
Transabdominal transducer (3–5 MHz)
Transvaginal US transducer to help detect placental abnormalities and extrauterine pregnancies
Adjustable acoustic power output controls with output display standards
Freeze-frame capabilities and electronic callipers
Obstetric presets (software) to estimate gestational age
Capacity to print or store images
Regular maintenance and servicing, important for optimal equipment performance
In general, service delivery settings that will only conduct routine basic obstetric ultrasound will not require a machine with additional features such as Doppler or 3-D/4-D imaging.
A transvaginal transducer may also be useful in some examinations where an experienced provider is unable to visualise anatomy with a transabdominal transducer.
Results
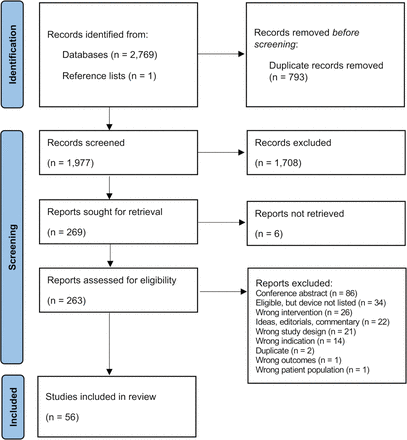
Literature searches for the scoping review identified 2770 citations, of which 793 duplicates were removed. Title and abstract screening of the remaining 1977 unique citations identified 269 citations which were potentially eligible. After reviewing full texts, 56 studies were included for analysis (figure 1). The most common reasons for exclusion included conference abstracts (86 studies), ultrasound device was not described (34 studies), or studies using an ineligible intervention (such as conventional ultrasound devices only) (26 studies). Six full texts were unable to be located. All data used in the results are publicly available online.18
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart of the screening process for scoping review.
Characteristics of included studies
Included studies were published between 2005 and 2021. Studies were conducted in 34 different countries across six regions (2 studies were conducted in multiple countries). High-income countries accounted for 24 studies (42.9%), lower middle income countries for 13 studies (23.2%), upper middle income for 9 studies (16.1%) and low-income countries for 8 studies (14.3%) (table 1). In terms of geographical regions, 16 studies (28.6%) were from sub-Saharan African countries, followed by Latin American and Caribbean countries (13 studies, 23.2%). The country with the highest number of studies was the USA (10 studies, 17.9%).
Number of studies per World Bank (2021) income level
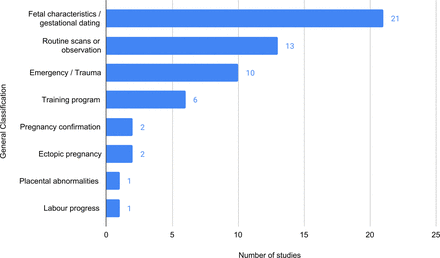
Cross-sectional study designs were most common (15 studies, 26.8%), followed by prospective or retrospective cohort studies (11 studies, 19.6%) and a single study using a case-control study design. The remaining 29 studies were pilot, field or validation studies. Studies were most commonly using portable ultrasound devices for transabdominal assessment (36 studies, 64.3%). Other studies related to training programmes for portable ultrasound use in pregnancy-related indications (9 studies, 16.1%), using portable devices with transvaginal ultrasound only (8 studies, 14.3%), and studies where existing ultrasound devices were modified, such as attaching a motor to a probe to allow for remote control of an ultrasound device (3 studies, 5.4%). In total, 21 studies related to assessment of fetal characteristics or performing gestational dating (37.5%). Other studies used portable ultrasound for routine antenatal scans or clinical observations (13 studies, 23.2%) and ultrasound use in emergency/trauma situations involving pregnant women (10 studies, 17.9%) (figure 2).
Studies classified by their main objective in using a portable ultrasound.
The 56 studies used 27 different portable ultrasound models, from 17 manufacturers (table 2). Nearly half used a device produced by SonoSite, with the most common being the SonoSite M-Turbo (10 studies, 17.9%) followed by the General Electric (GE) VScan (8 studies), SonoSite Titan and Micromaxx (4 studies each), and GE Voluson i (three studies). One device, the Enlace Hispano Americano de Salud Healthy Pregnancy Kit device, was described in two studies but does not appear to be commercially available.19 20
Number of studies, stratified by manufacturer and model of portable ultrasound device
Of the 56 studies, 47 (83.9%) primarily focused on pregnant women as participants, and 9 studies (16.1%) collected data related to staff members who participated in portable ultrasound training programmes. The 47 studies involved pregnant women without specific restrictions (32 studies), pregnant women who presented with vaginal bleeding (5 studies) or women with ectopic or clinically high-risk pregnancies (10 studies). For the nine studies that reported data on staff members being trained in portable ultrasound use, these involved multiple groups of health professionals (four studies), physicians only (two studies), nurses/midwives only (two studies) and medical students (one study).
Most studies (53 studies, 94.6%) were conducted in the antenatal period, though 2 were intrapartum and 1 was both antenatal and intrapartum. Of those 53 studies in the antenatal period, 10 were in the first trimester only, 5 in the second trimester only, 5 in the third trimester only and 5 across both second and third trimesters (the remaining 28 studies did not specify the pregnancy term period). Studies were conducted in outpatient antenatal care settings (30 studies, 53.6%), inpatient (24 studies, 42.9%) and community settings, such as local marketplaces or ‘field investigations’ (8 studies, 14.3%). One study assessed portable ultrasound in the context of telemedicine, and one study did not describe the setting.
Accuracy of portable ultrasound devices
A total of 21 studies related to portable ultrasound use for assessment of fetal characteristics and/or gestational age estimation, though only 10 of these formally validated a portable device against a conventional ultrasound. Findings from these 10 studies—including study design, objective, devices used and key findings—are presented in table 3. The devices used in these 10 studies were the GE VScan (4 studies); GE Logiq i (1 study); Konted Gen 1 C10R (1 study); Mindray DP-10 (1 study); Siemens Accuson 10 (1 study); SonoSite M-Turbo (1 study); and SonoSite Titan (1 study). These validation studies investigated device accuracy with regards to fetal biometric measurements such as biparietal diameter and femur length, as well as fetal number, fetal lie, gestational age, placental location, small or large for gestational age. The studies were conducted with women across a range of gestational ages. Of these 10 studies, 9 reported that the portable ultrasound device was partially or fully validated.
Characteristics and findings from studies comparing portable ultrasound devices against conventional ultrasound for assessing fetal characteristics and gestational age estimation
Commercially available portable ultrasounds
Web searches identified 106 portable ultrasound devices made by 26 different manufacturers (online supplemental table S8). The majority were produced in China, and prices ranged from US$1190 to US$30 000. Devices ranged in weight from 0.9 kg to 13.0 kg and battery life was from 40 min up to 8 hours. Identified devices were a mix of handheld devices with either wired or wireless connection to a user’s device (typically a phone or computer), or laptop-style portable ultrasound devices.
Supplemental material
Where sufficient data were available, we compared available devices against the requirements identified in WHO’s antenatal care recommendations for ultrasound devices (box 1). Though we did not have complete data on all identified devices, it was common for identified devices to have a transabdominal transducer, greyscale imaging capabilities, adjustable acoustic power output controls, freeze-frame capabilities, the capacity to store and print images and obstetric presets. For most devices, information was not available on whether regular servicing and maintenance was offered, and it was less common for transvaginal transducers to be available.
Discussion
Summary of main findings
This scoping review identified 56 studies related to the use of portable ultrasound devices in obstetric care, more than half of which were in LMICs. The review found that 27 portable ultrasound devices (from 17 manufacturers) had been formally evaluated in the peer-reviewed literature. These studies most commonly related to abdominal assessment using a portable ultrasound device, though studies relating to transvaginal ultrasound assessment and training programmes for healthcare workers on using portable ultrasound were also identified. Our results found that only 10 studies formally validated portable ultrasound devices against a conventional ultrasound device. Four studies assessed accuracy of gestational age estimation, while six studies assessed accuracy of selected fetal biometry measures, which can be used in estimating gestational age. These 10 studies incorporated 7 devices, with which only 6 were described as valid compared with their conventional counterpart. By comparison, 102 portable ultrasound devices are currently commercially available. While many of the available devices are promising in terms of function, portability and affordability, we identified no validation studies for the majority of commercially available devices.
Strengths and limitations
This review was conducted in accordance with a prespecified protocol, and in line with current scoping review methodological guidance.10–12 We searched a wide range of sources using robust search strategies, and studies were screened and extracted in duplicate and verified. The scoping review was augmented by additional web searches of ultrasound manufacturers, providing useful corollary information on the commercial availability of portable devices. However, some limitations must be acknowledged. Despite our best efforts, we were unable to locate six potentially eligible studies, which may have impacted the findings of this review. Also, some of the included studies required extensive discussions in the review team regarding the study design, intervention and what fetal measurements had been evaluated. We aimed to mitigate this through using operational definitions for study classification and data extraction, though this was challenging for some studies that were poorly reported. While nine studies were identified in which portable ultrasound devices were determined to be valid, it is possible that validation studies in other settings or populations may find different results. Also, sample sizes for these studies were not large—up to 251 women, and including a proof-of-concept study in a single woman.21 Hence, we consider it likely that further studies will be required for these devices also. It is important to acknowledge that ultrasound manufacturers may have conducted formal validation for portable ultrasound devices, but that these may not be available in the public domain. However, we consider it critical that any such studies should be made publicly available in the peer-reviewed literature, so that clinicians, administrators and policy-makers can appropriately scrutinise their accuracy and reliability.
Interpretation
This is the first review specifically examining the use of portable ultrasound devices for use in pregnant women. A 2016 review by Becker et al investigated portable ultrasound use across multiple health topics, identifying only three studies on pregnancy-related indications.9 Our review identified a higher number of studies, probably reflecting that a number of portable devices have entered the market since 2016, with an associated increase in research interest. It was noteworthy that over half of identified studies were conducted in LMICs, likely reflecting that this innovative technology is promising for limited-resource settings.
The large number of devices commercially available is consistent with expansion of this technology in recent years. However, only 27 of these devices have had been formally evaluated through some form of peer-reviewed research regarding their accuracy, feasibility, reliability or acceptability. In their 2019 commentary on medical device regulation, Charlesworth and van Zundert argued that while medical device manufacturers may posit that it is too costly, time-consuming, and impractical to generate evidence on devices from large studies, primary research is undeniably critical to ensuring that large-scale implementation will be beneficial.22 Relatedly, a major finding from this review is that further research on portable ultrasound devices—in particular their accuracy and acceptability when used in antenatal care contexts—are needed to guide decision-making around selection and procurement of ultrasound models.
Since 2016, WHO has recommended that all women should have an ultrasound prior to 24 weeks’ gestation; however, the coverage of ultrasound use remains limited in many countries.7 8 Findings of this review can be useful to maternity care clinicians, programme administrators and policy-makers who are seeking to identify reliable, affordable and portable ultrasound systems to use in their settings. However, available information was insufficient for most models, and only 10 had been formally validated for fetal biometry measures. In order to respond to this knowledge gap, and the growing number of commercially available devices, further peer-reviewed studies into portable ultrasound devices for obstetric use are required. These studies would ideally be independent (free from any financial bias or incentives from device manufacturers); use a diagnostic accuracy design (or similar) for routine fetal biometry measures; assess promising handlhed devices against a standard control; and be peer reviewed and publicly available. It is hoped that these studies would demonstrate convincingly that handheld devices perform as well as conventional ultrasound systems used in obstetrics.
Conclusion
A large number of portable ultrasound devices for obstetric use are commercially available; however, there is limited peer-reviewed research that has formally assessed how these devices perform against conventional ultrasound machines. Findings from this review, combined with future studies that assess the accuracy and validity of new technologies, can help support safe and effective implementation of portable devices, particularly for limited-resource settings where access to obstetric ultrasound is limited.
Data availability statement
Data are available in a public, open access repository.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
Acknowledgments
Lorena Romero (an information specialist) helped with the initial design of the search strategy. Anna Shalit and Lauren Vallely (medical students) helped with the initial title/abstract screening.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors AJE developed the review protocol and data extraction tools. AJE and SM (an information specialist) developed the search strategy. AJE, EF and SA conducted title/abstract and full-text screening and data extraction—conflicts were resolved by either EF or SA depending on which was not involved in the initial decision. AJE prepared the first draft of the analysis, which was reviewed by all authors and revised following their input. AJE is responsible for the overall content as guarantor for this review. All named authors contributed to the writing of this manuscript. The authorship team is comprised of medical doctors, a maternal and public health researcher, and an information specialist.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.