Article Text
Abstract
Introduction Hepatocellular carcinoma (HCC) remains a major clinical problem as more than half of these cases recur after radical resection. Natural killer (NK) cells are at the forefront of the innate immune system and attack microcarcinomas and circulating tumour cells. The objective of this study was to evaluate the feasibility and toxicity of peripheral blood CD34+ stem cell-derived NK cell infusion after radical hepatectomy for HCC.
Methods and analysis This is an open-label, single-arm, single-centre phase I study. Patients who have undergone initial hepatectomy for HCC with three or more risk factors for recurrence (≥10 ng/mL of Alpha fetoprotein (AFP), ≥360 mAU/mL of PIVKA-II, multiple tumours and ≥3 peripheral blood circulating tumour cells) will be enrolled and be treated with three peripheral blood CD34+ stem cell-derived NK cell infusions every 3 months. The primary endpoint will be safety assessment including the type and severity of adverse events, frequency of occurrence and duration of occurrence. The secondary endpoints will include survival, effect of immune response and clinical laboratory test results.
Ethics and dissemination Ethical approval of the trial was obtained from the Certified Committee for Regenerative Medicine Hiroshima University in Japan. The trial results will be shared with the scientific community at international conferences and by publication in a peer-reviewed journal.
Trial registration number jRCTb060200020.
- IMMUNOLOGY
- Hepatobiliary surgery
- SURGERY
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Granulocyte colony stimulating factor administration and apheresis must be performed to yield CD34-derived stem cells.
Activated natural killer cells are created from CD34-positive stem cells using cytokines.
Patients receive this protocol treatment every 3 months for a total of three times.
The feasibility study design with consequent small sample size does not allow for accurate survival analysis.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy and the third leading cause of cancer death worldwide.1 Although HCC can be cured by hepatic resection, HCC recurrence occurs in almost 70% of patients within 5 years.2 Novel immune checkpoint inhibitor therapies for recurrent and advanced HCC have been substantial and reported to have good results.3 However, no standard adjuvant therapy has yet been established with proven efficacy in preventing recurrence.
Natural killer (NK) cells are the first defence of the cancer immune system and can kill target cells without prior sensitisation based on inhibitory receptor recognition (missing-self hypothesis) and activated receptor recognition.4 NK cell activity is significantly decreased in patients with liver cirrhosis,5 after hepatectomy6 and during chemotherapy.7 In our previous studies, liver mononuclear cells derived from donor liver perfusate contained many NK cells that have vigorous cytotoxicity against hepatoma cells; these NK cells express tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) after in vitro stimulation.5 6 8 We have undertaken a pilot clinical trial for an adoptive immunotherapy approach, using lymphocytes extracted from the liver allograft perfusate, which includes an abundance of NK cells that mount an anti-HCC response, in liver transplant recipients with HCC.9 10 However, since liver-derived NK cells can only be obtained from liver transplant donors, we developed a procedure to generate hepatic endogenous NK cell-like cells from peripheral blood CD34+ stem cells. This clinical phase I study aims to determine the feasibility of adoptive immunotherapy using peripheral blood CD34+ stem cell-derived NK cells to prevent HCC recurrence after curative hepatectomy.
Methods and analysis
Study design
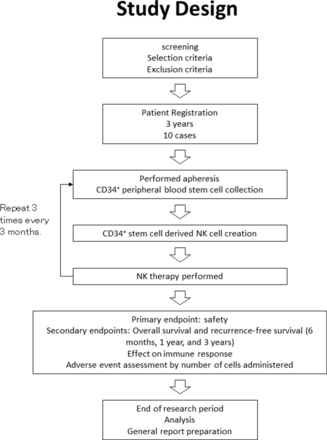
This clinical study is a phase I trial of an immunostimulatory therapy using peripheral blood CD34+ stem cell-derived NK cells to prevent recurrence of HCC after liver resection in high-risk liver cancer patients. The primary endpoint of this study will be safety, and it will be conducted as an open-label study to collect more safety information when NK therapy is administered. The study design is shown in figure 1. Patients have been enrolled since November 2021. The study is scheduled to run until March 2026.
Study design. NK, natural killer.
Inclusion criteria
Inclusion criteria were patients:
Who underwent initial hepatectomy for HCC.
Aged between 20 and 79 years.
With haemoglobin level of 100 g/L or greater and platelets of 109/L or greater.
Who have appropriate vessels for apheresis or who agree to catheter insertion in case of inappropriate vessels.
Who have three or more risk factors of: AFP (Alpha fetoprotein)>10 ng/mL, PIVKA-II>360mAU/mL, multiple tumours and circulating tumour cells >3 in the peripheral blood.
With performance status of 0 or 1.
With pathologically no tumour residue (R0).
Who have signed written informed consent.
Exclusion criteria
Exclusion criteria were patients:
Who have malignant disease other than HCC.
Are on steroids or immunosuppressive drug therapy.
Who have previously undergone hepatectomy.
Are not eligible for recombinant human granulocyte colony stimulating factor (rhG-CSF) or apheresis; (i) with allergy to rhG-CSF or are pregnant or possibly pregnant, (ii) with a history of coronary artery disease or cerebrovascular disease within the past 6 months, (iii) with heart disease (Ejection fraction (EF) <25%), lung disease, or renal disease requiring treatment, (iv) with neurological disorders, (v) with suspected myeloproliferative disorders such as leucocytosis and thrombocytosis, (vi) with leucocytes less than 3 × 10∧9/L or more than 10 × 10∧9/L and (vii) with a history of interstitial pneumonia.
Who have been hospitalised more than 6 weeks after surgery due to postoperative complications.
Deemed by researchers to be inappropriate participants.
Concomitant treatment with anticancer and immunosuppressive agents will not be used. However, the use of steroids as a pretreatment for cell administration and the use of immunosuppressive agents for the treatment of adverse events (AEs) (except for prophylactic measures for the occurrence of AEs) will not be stopped.
Sample size calculation
There were 37 patients in a recurrence risk group among 132 liver resections for initial liver cancer performed between January 2015 and December 2018. If about 30% of the patients would participate in the clinical trial, this would be 12 cases over 3 years. Considering the dropout cases at the time of enrolment, the target number of patients was set at 10.
Treatment
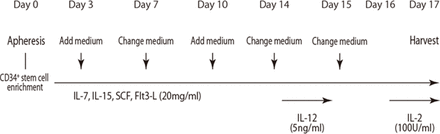
One to 3 months after the initial hepatectomy for HCC, patients received rhG-CSF (Filgrastim syringe, Kyowa Kirin) 400 μg/m2 subcutaneously once daily for 4–6 days, and peripheral blood CD34+ stem cell collection by apheresis was performed on the day of the last Filgrastim syringe administration. Lymphocyte fractions were extracted from the peripheral blood CD34+ stem cell-containing solution using the specific gravity centrifugation method. Lymphocytes derived from CD34+ stem cells in the peripheral blood were cultured with GMP-grade human interleukin (IL) 7 (Miltenyi Biotec), GMP-grade human IL-15 (Miltenyi Biotec), GMP-grade human stem cell factor (Miltenyi Biotec) and GMP-grade human Fms-like tyrosine kinase 3 ligand (Miltenyi Biotec) (all at a final concentration of 20 ng/mL), and human AB serum (5% final concentration). The culture method is involved adding the medium every 3 days and changing the medium every 7 days for a total of 17 days.
Three days prior to cell administration (day 14), GMP-grade human IL-12 (Miltenyi Biotec) was added to the cell culture medium at a final concentration of 5 ng/mL to activate NK cells. One day prior to cell administration (day 16), GMP-grade human IL-2 (Immunes, Kyowa Pharm) was added to the medium at a final concentration of 100 IU/mL. On the day of treatment, the cell suspension was collected, centrifuged, washed with saline and then suspended in albumin-containing saline and administered intravenously (figure 2). Protocol treatment is defined as from apheresis to the completion of peripheral blood CD34+ stem cell-derived activated NK cell administration. Patients will receive this protocol treatment every 3 months for a total of three times.
Cell processing protocol. IL, interleukin.
Follow-up and assessment of efficacy
The primary endpoint of the study will be to assess the safety of the treatment (type and severity of AEs, frequency of occurrence and duration of occurrence). The criteria for serious AEs will be as follows: haematological toxicity (anaemia, leucopenia, leucocytosis and thrombocytopenia), hepatic function abnormalities (elevated aspartate aminotransferase, alanine transaminase, alkaline phosphatase and T-Bil), electrolyte abnormalities (hyponatraemia, hypokalaemia, hypokalaemia, hypercalcaemia, hyperkalaemia and hypercalcaemia), non-haematological toxicity (general malaise, headache and fever), anorexia, nausea, diarrhoea, constipation, laryngeal oedema, mucositis, dyspnoea, hypoxia, pulmonary oedema, neuropathy, skin rash, allergic reaction, cytokine release syndrome and hypotension. The secondary endpoints will be the following: (1) overall survival and recurrence-free survival (1 and 3 years after surgery), (2) effect on immune response: evaluation of peripheral blood NK cell activity, (3) AE assessment by cell dose, (4) vital signs and (5) clinical laboratory test results.
Immunological assessment
All flow cytometry analyses will be performed on a BD FACS Canto II flow cytometer (BD Biosciences, San Jose, California, USA). To detect the surface phenotype, leucocytes will be stained with the following monoclonal antibodies: against CD3, TRAIL, NKG2D, CD69, CD226 and CD56. The data will be analysed using FlowJo software (Tree Star, Inc. Ashland, Oregon, USA). A 51Cr-release assay will be performed as previously described,5 using HepG2 tumour cells (ATCC) as targets. Briefly, 51Cr-labelled target tumour cells will be added for 4 hours at 37°C to effector cells in round-bottomed 96-well microtitre plates (BD Biosciences, Discovery Labware). The percentage of specific 51Cr release will be calculated as follows: % cytotoxicity=[(cpm of experimental release–cpm of spontaneous release)/(cpm of maximum release–cpm of spontaneous release)]×100. All assays will be performed in triplicate.
Safe evaluation and reporting of adverse effects
After cell administration, the investigator will record all AEs, whether or not considering to be related to cell administration. In the event of serious complications related to prolonged hospitalisation or death, the investigator will promptly report to the Committee for Regenerative Medicine and the Ministry of Health, Labor and Welfare. The principal investigator or sub-investigator will discontinue or suspend clinical research on the subject in the following cases: (1) if a positive result is found in the infectious disease test of the culture test material; (2) protocol treatment is no longer possible; (3) subject requests withdrawal of consent for participation in clinical research or (4) the occurrence of an AE is recognised, and the principal investigator judges that it is difficult to continue clinical research. Our protocol specifies that a clinical trial will be terminated as follows: (1) significant information regarding the quality, safety or efficacy of the cellular conditioning is obtained. (2) When it is deemed difficult to conduct the clinical research due to delays in case enrolment, frequent protocol deviations or other reasons. (3) When it is determined that there is a problem with the safety of the protocol treatment based on the evaluation by the Committee for Regenerative Medicine. (4) If, as a result of the evaluation of relevant information obtained from sources other than this clinical research, such as papers and conference presentations, it is determined that there is a problem with the safety of the protocol treatment, or that the continuation of the clinical research is no longer meaningful.
Statistics
The primary objective will be to determine the safety of this treatment. Safety assessment will include observation and recording of any grade of AEs according to the latest version of the NCI-CTC. As a rule, for items observed as continuous values, the number of examples, number of missing examples, mean (median), SD (quartiles) and range (minimum-maximum) will be calculated as summary statistics. For items observed as discrete values, the number of examples in each category and their percentages will be calculated as summary statistics. Statistical tests for the main analysis will be performed at a 5% significance level (two-sided).
Patient and public involvement
No patient involved.
Ethics and dissemination
This trial will be conducted in conformance with the principles of the ‘Declaration of Helsinki’. All patients must be given written informed consent to a member of the study team before inclusion in this study. The protocol is approved by the Certified Committee for Regenerative Medicine Hiroshima University, Japan and has been prospectively registered in Japan Registry of Clinical Trials.
A summary of the results will be provided the competent authority and the Certified Committee for Regenerative Medicine Hiroshima University within 1 year after the end of the trial. All subject records will be retained for 30 years following completion, termination or discontinuation of the clinical investigation. The results of the clinical trial will be published in a peer-reviewed journal.
An electronic case report form is made using REDCap Electronic Data Capture, where all data on patient eligibility, treatment cycles and clinical parameters will be collected by trained staff members in Hiroshima University Hospital. The clinical trial will be monitored approximately two times a year by an independent monitor.
Discussion
This trial will investigate whether adoptive immunotherapy using peripheral blood CD34+ stem cell-derived NK cells can be safely administered to HCC patients after curative resection. The primary endpoint will be to assess the safety of this treatment protocol. The secondary endpoints will be survival, recurrence-free survival, immunological assessment, side effects and laboratory data analysis.
There are several methods of NK cell-based cell therapy, which can be categorised into those using peripheral blood primary NK cells, apheresis products and umbilical cord blood.11 Peripheral blood NK cells are easy to use and have been used for a long time, but their cellular differentiation is mature, it is difficult to obtain further enhancement of their function and their life span is short. In addition, NK cell activity in patients with liver cirrhosis and HCC is already exhausted,12–14 which is not desirable for cell therapy. Conversely, the method of developing NK cells from haematopoietic stem cells by apheresis is not affected by the primary disease, and since NK cells are manufactured from undifferentiated cells, they can be expected to be active in the body. For a combination of others, T cells need to be removed because of the risk of graft-versus-host-disease and the high possibility of rapid elimination of the administered NK cells.15
Phase I clinical trials of cell products stimulated and cultured from autologous or allogeneic peripheral blood lymphocytes have been conducted in lymphoma, breast cancer,16 gastric cancer,17 gastrointestinal cancer,18 non-small cell lung cancer19 and other cancer types, and the safety of cell therapy has been uniformly reported, although the formulation process has differed. However, phase II efficacy studies have not shown efficacy against these cancer types.15 Under these circumstances, postoperative adjuvant therapy with autologous peripheral blood-derived lymphocytes, as adjuvant therapy after curative resection for HCC has been shown to be effective in preventing recurrence in randomised controlled trials in Japan and overseas.20 21 These studies showed prolonged recurrence-free survival but not overall survival. Recently, however, multiple doses of autologous peripheral blood-derived activated lymphocytes were reported to prolong recurrence-free survival and overall survival as adjuvant therapy after local treatment, including surgery.22 From these studies, HCC would be a cancer type that is expected to benefit from cell therapy.
We will thus analyse the safety and potential antitumour effects of the therapy in this clinical study. We expect that these findings will lead to future phase II/III trials.
Ethics statements
Patient consent for publication
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
References
Footnotes
Contributors MO, TK, YK and HO conceived the idea of the study. RK, KY, KU, NT and TH developed the statistical analysis plan and conducted statistical analyses. YI, KS, KI, RN, MD, JP and MN manufactured samples. TY, TI, MI, HA, NT, SK, HT and KI contributed to the interpretation of the results. MO drafted the original manuscript. MO supervised the conduct of this study. All authors reviewed the manuscript draft and revised it critically on intellectual content. All authors approved the final version of the manuscript to be published.
Funding This study was supported by AMED under Grant Number JP22fk0210108 (Hideki Ohdan) and JSPS KAKENHI Grant Number JP20K09104 (Masahiro Ohira).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.