Article Text
Abstract
Objectives To describe the nature, frequency and content of non-vitamin K oral anticoagulant (NOAC)-related events for healthcare professionals sponsored by the manufacturers of the NOACs in Australia. A secondary objective is to compare these data to the rate of dispensing of the NOACs in Australia.
Design and setting This cross-sectional study examined consolidated data from publicly available Australian pharmaceutical industry transparency reports from October 2011 to September 2015 on NOAC-related educational events. Data from April 2011 to June 2016 on NOAC dispensing, subsidised under Australia’s Pharmaceutical Benefits Scheme (PBS), were obtained from the Department of Health and the Department of Human Services.
Main outcome measures Characteristics of NOAC-related educational events including costs (in Australian dollars, $A), numbers of events, information on healthcare professional attendees and content of events; and NOAC dispensing rates.
Results During the study period, there were 2797 NOAC-related events, costing manufacturers a total of $A10 578 745. Total expenditure for meals and beverages at all events was $A4 238 962. Events were predominantly attended by general practitioners (42%, 1174/2797), cardiologists (35%, 977/2797) and haematologists (23%, 635/2797). About 48% (1347/2797) of events were held in non-clinical settings, mainly restaurants, bars and cafes. Around 55% (1551/2797) of events consisted of either conferences, meetings or seminars. The analysis of the content presented at two events detected promotion of NOACs for unapproved indications, an emphasis on a favourable benefit/harm profile, and that all speakers had close ties with the manufacturers of the NOACs. Following PBS listings relevant to each NOAC, the numbers of events related to that NOAC and the prescribing of that NOAC increased.
Conclusions Our findings suggest that the substantial investment in NOAC-related events made by four pharmaceutical companies had a promotional purpose. Healthcare professionals should seek independent information on newly subsidised medicines from, for example, government agencies or drug bulletins.
- public health
- medical ethics
- medical education & training
- quality in health care
- haematology
- cardiology
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
We used a unique database of more than 100 000 industry-sponsored events for healthcare professionals to examine the frequency and characteristics of events related to the non-vitamin K oral anticoagulants (NOACs).
We compared the frequency of events with whole-of-population, administrative data on NOAC dispensing, but could not assess causal links between pharmaceutical industry spending on events and prescribing.
We searched for NOAC-related events using a set of keywords; however, some events may not have been captured due to limited detail within the company’s description of sponsored event.
We conducted an analysis of the content presented at two sponsored events; however, this analysis was limited to large-scale events with readily accessible content, and may not be representative of all events.
Introduction
Between October 2011 to September 2015, 47 pharmaceutical companies sponsored more than 116 000 events with over 3 million attendances by Australian doctors, nurses, pharmacists and specialists. Sponsors provided attendees with free dinners, lunches, refreshments, beverages, and some travel and accommodation to overseas locations; amounting to over $A286 million.1 Exposure to information presented at these types of events is associated with greater numbers of prescriptions.2 There is also an association between increased prescribing of brand-name medications and exposure to industry-sponsored events, particularly those serving meals and beverages.3
Three non-vitamin K oral anticoagulants (NOACs)—rivaroxaban (Xarelto), dabigatran (Pradaxa) and apixaban (Eliquis)—were first approved for use in Australia by the Therapeutic Goods Administration (TGA) between 2008 and 2011 and listed on the Pharmaceutical Benefits Scheme (PBS), Australia’s national drug subsidy programme between 2009 and 2012.4–7 Soon after the expansion of PBS subsidy to include the indication of thromboprophylaxis in non-valvular atrial fibrillation in 2013, use of the NOACs increased exponentially.8 In 2015, 1 604 242 PBS-subsidised NOAC prescriptions were dispensed for 188 130 patients.7 Warfarin, an older and well-studied anticoagulant, began a gradual decline in its prescribing in 2013. However, the NOACs are an expensive alternative to warfarin. Between 2016 and 2017, the Australian government spent $A107 980 701 on rivaroxaban, almost six times the amount spent on warfarin, $A18 701 242.9
Systematic reviews and meta-analyses on the efficacy and safety of the NOACs versus warfarin in atrial fibrillation and venous thromboembolism—for which these oral anticoagulants are primarily indicated—found that the NOACs were marginally more effective than warfarin, with no significant difference in safety.10–14 However, evidence of falsified data and other violations within clinical trials of apixaban and rivaroxaban cast doubt on the accuracy of these findings.15 16 Concerns have also been raised over the extensive pharmaceutical industry ties among the authors of the NOAC clinical trials.17 The NOACs have also been associated with major bleeding events, particularly increased gastrointestinal bleeding.18 19 A retrospective analysis of the Food and Drug Administration (FDA) Adverse Event Reporting System database found that, although bleeding events were more frequent with warfarin compared with dabigatran, 15% of the events reported for dabigatran resulted in fatalities, versus only 7% for warfarin.20 Furthermore, registered reversal agents do not yet exist for rivaroxaban and apixaban, meaning the rapid reversal of their anticoagulant effects in life-threatening bleeding emergencies is often not possible.21–23
It has been anecdotally reported that the manufacturer of dabigatran, Boehringer Ingelheim, heavily promoted the drug in industry-sponsored events targeted towards prescribers around the times of its PBS-listings by the Australian government,24 raising questions about the role of pharmaceutical company promotional activities in the exponential increases in NOAC prescribing. In the USA, pharmaceutical industry payments directly to physicians have previously been associated with higher NOAC prescribing within hospital referral regions,25 but direct payments to physicians are just one way that the pharmaceutical industry interacts with prescribers. Industry-sponsored events as a source of promotion have not been examined. Australian transparency databases provide a unique opportunity to examine the potential role of pharmaceutical industry sponsorship of educational events for healthcare professionals in NOAC promotion.26 Medicines Australia, the pharmaceutical industry trade organisation, requires member companies to submit reports on sponsorship of events for physicians and other healthcare professionals, including spending on food and beverages, trade displays, sponsorship of healthcare professional attendance, speaker fees and other associated costs. Here, we use these reports to describe the nature, frequency and content of NOAC-related events for healthcare professionals sponsored by the manufacturers of the NOACs in Australia. The secondary objective is to compare these data to NOAC dispensing in Australia.
Methods and analysis
Study design and setting
This cross-sectional study examined previously consolidated data from publicly available Australian pharmaceutical industry transparency reports from October 2011 to September 2015. We extracted data on payments for educational events and described these events. PBS data on NOAC dispensing from April 2011 to June 2016 were also obtained.
Data sources
Educational events database
Previously, 301 pharmaceutical company reports by 42 pharmaceutical companies on educational events were downloaded from the Medicines Australia website (https://medicinesaustralia.com.au/).1 These PDF reports were converted into Excel files using free, online file conversion software. The files were cleaned to resolve any discrepancies as a result of the conversion process and to remove text from columns that should have only contained numerical values. Following this, the data were consolidated into one Excel file and made publicly available for research and public use (https://researchdata.ands.org.au/pharmaceutical-industry-funded-sept-2015/941218). The dataset included the name of the sponsoring company, a brief description of each event, the event venue and date (month, year), the number and professional status of attendees, the type of support or sponsorship provided by the company, the cost of any food and beverages provided and the total cost paid by the company.26 Information on attendees reflected attendances rather than discrete individuals, as one individual may have attended multiple events.
We focused on events funded by Bayer, Pfizer, Bristol-Myers Squibb (BMS) and Boehringer Ingelheim; the manufacturers of rivaroxaban (brand name Xarelto), apixaban (Eliquis; co-manufactured by both BMS and Pfizer) and dabigatran (Pradaxa), respectively.
NOAC dispensing data
Publicly available reports on the dispensing of PBS-listed drugs were obtained from two sources: (1) Australian Government Department of Health Date of Supply Reports, available July 2013–July 2018 (http://www.pbs.gov.au/info/statistics/dos-and-dop/dos-and-dop) and (2) the Department of Human Services PBS Item Reports, available January 1992–September 2018 (http://medicarestatistics.humanservices.gov.au/statistics/pbs_item.jsp). Reports contained information on the number and costs of dispensed PBS-listed prescription medications. Data on NOAC dispensing (April 2011–July 2018) were extracted using PBS item codes. We used the Date of Supply reports for the period July 2013–July 2018 and supplemented our data with the Item Reports for the period April 2011–June 2013. Although PBS Item Reports were available for the entire period of interest, we preferentially relied on Date of Supply Reports; PBS Item Reports are based on the date of processing of the claim for reimbursement rather than the date of supply (dispensing) of the medicine from the pharmacy, leading to delays in recording and misleading peaks and troughs associated with periods of bulk processing.27
As changes in regulatory approval and subsidy influence both prescribing and marketing, we reviewed TGA and Pharmaceutical Benefits Advisory Committee (PBAC; the body responsible for recommending the listing of new medicines on the PBS) decisions on NOAC listing during the study period. We extracted the details of major decisions (including changes to listing such as expansion of indication or addition of new doses, and rejected applications) from publicly available Australian Public Assessment Reports for prescription medicines, NOAC product information documents and PBAC reports.7 28 29
Data coding and extraction
Educational events database
In order to identify events related to the NOACs, we confirmed that the manufacturers of these drugs made no others for the same indications. We extracted events sponsored by the NOAC manufacturers from the Educational Events Database and identified NOAC-related events by searching event descriptions for NOAC-related keywords and keyword combinations (see online supplementary materials table S1). For these events, we extracted information on the profession of healthcare professionals attending the event (including general practitioners, haematologists, cardiologists, nurses, registrars and pharmacists), the location of the events, food and beverages provided, and type of event. We used the event description to categorise the type of event as organised meetings (such as conferences and seminars), in-services/staff training sessions, journal clubs, grand rounds and workshops. We also categorised the location of the event into clinical settings (such as hospitals, medical centres and clinics) and non-clinical settings (such as restaurants, hotels and conference centres) according to the event venue reported by the company.
Supplemental material
Content of educational events: illustrative case studies
We selected two major events that were sponsored by the manufacturers of the NOACs as illustrative case studies: the European Society of Cardiology (ESC) Congress 2015 and the 19th Congress of the European Haematology Association (EHA). We chose these events because (1) all four NOAC-manufacturers sponsored at least one of the events, (2) they were major international events in cardiology and haematology, respectively, and (3) information on the content of these events was publicly available. We used the Educational Events database to extract information on the sponsorship of the event by each company, including cost, purpose and profession of the healthcare professional recipients or attendees. We conducted an online search for additional information on the event, such as copies of presentations (posters, PowerPoint, videos and so on), programme and other materials provided to attendees; and related articles and commentary. We extracted details on: the identity of the speakers and presenters, including any declared conflicts of interests; titles and content of presentations and posters; claims about the NOACs, including efficacy, superiority over other treatments, adverse events, indications for use (including unapproved uses) and target patient populations; and sponsorship of presentations or posters by the NOAC manufacturers. The recovered content was summarised into descriptive case studies for each congress. Particular attention was paid to satellite symposia, as bias in sponsored symposia has been previously identified.30
NOAC dispensing data
Data on the number of NOAC prescriptions dispensed per month were extracted and graphed over time. The time period of these graphs (April 2011–June 2016) included the reporting period of the Educational Events Database, with an additional 6 months following and prior to this period to account for promotion in the lead-up to or following changes in prescribing. As apixaban was first PBS-listed in January 2012, no dispensing data were available prior to this date. The number of industry-sponsored events per quarter by a particular NOAC-manufacturer was plotted against the dispensing of that company’s NOAC. Major changes in PBS subsidy occurring within the period of the Educational Events Database were also indicated on these graphs. We focused on the number of events sponsored by each company in the period prior to and following the PBS-listing of each drug for the prevention of stroke or systemic embolism in patients with non-valvular atrial fibrillation. This listing was chosen as it had a substantial impact on prescribing.7 8
Analysis
We created frequency tables for NOAC-related event characteristics, including the frequencies and percentages of events containing each type of attendee, median costs (overall, and food and beverages only) per person and per event, and the percentages of events and costs of NOAC-related events for each company. As the data were not normally distributed, we present median with IQR instead of mean. We excluded values equal to zero when calculating median figures in order to prevent obtaining lower than true values. All costs are expressed in Australian dollars. Microsoft Excel was used for all analyses and figures.
Patient or public involvement
No patients or members of the public were involved in this study.
Results
Overview of NOAC-related educational events
Table 1 summarises the key characteristics of the events. Between October 2011 and September 2015, a total of 15 463 educational events were sponsored by the four manufacturers of the NOACs, of which 18% (2797) were NOAC-related. About half of all NOAC-related events (51%) were sponsored by Pfizer and BMS, the manufacturers of apixaban.
Summary of characteristics of non-vitamin K oral anticoagulant (NOAC)-related events from educational events database
Attendees
In total, 89 491 attendances were recorded at NOAC-related events. The median number of attendees per event was 20 (IQR=12–28). Among all NOAC-related events, 1174 events (42%) were attended by general practitioners, 977 events (35%) were attended by cardiologists, 635 events (23%) were attended by haematologists and 596 events (21%) were attended by nurses (table 1). Cardiologists were present at 70% of NOAC-related events hosted by Boehringer Ingelheim.
Payments
In total, $A10 578 745 was spent on all NOAC-related events (table 1). This included funding for venue hire, invitations, audio-visual equipment hire, accommodation and travel costs for selected delegates, congress registrations, meals and beverages, parking fees, honorarium fees, writing materials for attendees and third-party event organiser fees (such as for filming, banners, photography and speaker liaisons). For three of the four companies, about a quarter or more of their total event spending was dedicated towards funding NOAC-related events: 38% ($A3 290 443) by Boehringer Ingelheim, 29% ($A3 787 717) by BMS, 24% ($A1 959 467) by Bayer and 8% ($A1 541 118) by Pfizer (table 1). The median cost per NOAC-related event sponsored by Boehringer Ingelheim was $A2232 (IQR=$A1689–$A2984), more than four times the median amounts of the other manufacturers.
All four companies provided meals and beverages at their NOAC-related events, with 85% (2385/2797) of all NOAC-related events supplying food to attendees (table 1). Moreover, $A4 238 962 was spent by all NOAC-manufacturers on meals and beverages alone. Boehringer Ingelheim contributed the most to this amount, with $A2 509 919, mainly towards dinners and alcohol—two to three times the expenditure of the other companies. The median costs of food and beverages per person were highest for Boehringer Ingelheim at $A66 (IQR=$A51–$A80), and lowest for Pfizer at $A12 ($A9–$A25).
Locations and settings
More than half (52%; 1450/2797) of NOAC-related events were held in clinical settings such as hospitals and medical centres, with the remainder held in non-clinical settings such as restaurants, cafés, bars, clubs, and hotel resorts. However, 98% (613) of Boehringer Ingelheim’s sponsored events were held in non-clinical venues. The majority of events were held in Australia (87%; 2441), although 40% (277) of BMS’s sponsored events were held overseas (table 1).
Type of event
A little more than half (55%; 1551/2797) of sponsored events were identified as organised meetings, with the event type unspecified for 12% (341) of events (table 1). Only 39% (270/685) of events by BMS and 26% (195/747) of events by Pfizer had durations of 1 hour or less. Durations of events sponsored by Boehringer Ingelheim and Bayer were not provided.
NOAC dispensing
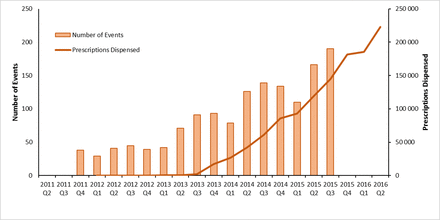
Figures 1–3 depict quarterly dispensing of rivaroxaban, apixaban and dabigatran, respectively, versus the frequency of events sponsored by each drug’s manufacturer over the time period of the Educational Events Database. TGA and PBAC decisions regarding NOAC regulatory approval and subsidy are presented in online supplementary tables S2 and S3, respectively.
Quarterly number of rivaroxaban prescriptions dispensed and non-vitamin K oral anticoagulant-related educational events sponsored by Bayer, April 2011–June 2016.
Quarterly number of apixaban prescriptions dispensed and non-vitamin K oral anticoagulant-related events sponsored by Pfizer and Bristol-Myers Squibb, April 2011–June 2016.
Quarterly number of dabigatran prescriptions dispensed and non-vitamin K oral anticoagulant-related events sponsored by Boehringer Ingelheim, April 2011–June 2016.
PBS dispensing data are subject to seasonality, with increased utilisation toward the end of the year followed by a trough at the start of the following year.23 This seasonality is due to the effect of the PBS Safety Net, a scheme that provides people with high medicine costs (over a certain threshold) with PBS medicines at reduced price for the remainder of the calendar year. This encourages individuals to buy extra quantities toward the end of the year (stockpiling) before prices reset in the new year. There was also a seasonal decline in the number of educational events in the summer holidays (December/January).
Dispensing of all three NOACs was low prior to PBS subsidy for the prevention of stroke or systemic embolism in non-valvular atrial fibrillation on 1 August 2013 (rivaroxaban), and 1 September 2013 (apixaban and dabigatran), after which utilisation increased rapidly. The change in subsidy was also associated with an increase in the number of NOAC-related events.
Rivaroxaban dispensing was low and relatively stable prior to the extension of subsidy, averaging 1184 dispensings per month between April 2011 and December 2012, before gradually increasing to 5426 in July 2013 (figure 1). Dispensing increased more than threefold between July and August 2013 with the change in subsidy (from 5426 to 17 222 dispensings) and another fourfold over the following year (to 68 719 dispensings in July 2014). Use continued to increase over the remainder of the study period, although at a slower rate, with a 44% increase in rivaroxaban dispensing between August 2014 and July 2015. The number of events sponsored by Bayer increased from 103 in the year preceding the listing (August 2012–July 2013) to 261 over the following year. The number of events was lowest between October 2011 and March 2013, with a median of 21 events per quarter (IQR: 16.3–22.8). This doubled to 42 events/quarter (IQR: 40–45) around the time of subsidy (April 2013–March 2014) and continued at a higher rate (80 events/quarter, IQR: 64–84) throughout the remainder of the study period.
Apixaban use averaged 69 prescriptions per month prior to the extension of subsidy in September 2013 (figure 2). Dispensing increased more than 10-fold in the year following subsidy (September 2013–August 2014), from 1972 to 20 282 dispensings per month, and continued to increase, more than doubling to 47 476 in August 2015. There were 222 NOAC-related events sponsored by BMS and Pfizer, manufacturers of apixaban, in the year before subsidy (September 2012–August 2013), increasing to 420 events over the following year. As with Bayer, the number of events was lowest between October 2011 and March 2013, with a median of 40 events per quarter (IQR: 38.3–41.8), and increased around the time of subsidy, with 85 events/quarter (IQR: 40–45) between April 2013 and March 2014. Apixaban-related events continued to increase throughout the study period, to 190 events in 2015 Q3.
On average, 47 dabigatran prescriptions were dispensed per month over the 2 years prior to the change in subsidy in September 2013 (figure 3). Between August and September 2013, dispensing increased from 76 to 8100 prescriptions, further increasing to 24 705 prescriptions in December 2013 before plateauing. There were 80 NOAC-related events sponsored by Boehringer Ingelheim before subsidy (September 2012–August 2013) increasing to 218 events over the following year. Boehringer Ingelheim sponsored a median of 21 events per quarter (IQR: 16–31) between October 2011 and March 2013, increasing to a peak of 66 events around the time of subsidy (2013 Q4), and continuing at a median rate of 49 events per month (44–58) for the remainder of the study period.
Content of educational events: illustrative case studies
Boxes 1 and 2 summarise two major international events that were sponsored by the NOAC manufacturers. The European Society of Cardiology (ESC) Congress 2015, attended by over 32 000 attendees, was sponsored by Boehringer Ingelheim ($A214 033 sponsorship), Pfizer ($A100 315) and Bayer ($A36 615). In 2014, BMS and Bayer spent $A192 080 and $A12 739, respectively, on the 19th Congress of the European Haematology Association (EHA), held in Milan, Italy with almost 11 000 people in attendance. Our analysis revealed a high number of NOAC-related presentation sessions and sponsored satellite symposia, often involving speakers with financial ties to the NOAC manufacturers, and the presence of material of a promotional character. Events promoted NOACs for unapproved indications and emphasised a favourable risk-benefit profile.
European Society of Cardiology (ESC) Congress (2015)
In August 2015, Boehringer Ingelheim sponsored 19 cardiologists and Pfizer sponsored seven cardiologists to attend the ESC Congress 2015 in London. Boehringer Ingelheim sponsored the healthcare professional attendees with $A214 033 in total (on average, $A11 265 per person), and Pfizer with $A93 215 (on average, $A13 316 per person). Payments included business class flight fares, accommodation, congress registration, meals, taxi fares and public transport fares for selected delegates. Pfizer also sponsored three dinners for 33 cardiologists for an additional $A3972, or $A120 per person. Bayer also provided $A36 615 sponsorship for the event. This event included 46 NOAC-related poster presentations and 14 NOAC-related satellite symposia.44
Eleven of the 46 posters (24%) were funded by the manufacturers of the NOACs and 28% (13/46) were coauthored by at least one person who worked for one of the manufacturers of the NOACs. Poster content included unapproved indications for the NOACs such as improvements in atherosclerosis and osteoporosis, reduction of smooth muscle dysfunction and use during catheter ablation for atrial fibrillation. Posters also favourably compared one NOAC to another and were more likely to be sponsored by the maker of the favoured NOAC. All speakers at the 14 satellite symposia had previously received honoraria, study grants, speaker fees or undertaken consultation for the manufacturers of the NOACs. Boehringer Ingelheim sponsored seven of these symposia, Bayer sponsored four, and BMS and Pfizer sponsored three.
During the conference, two complaints were filed by attendees.45 46 One complainant claimed that Boehringer Ingelheim had discussed off-label (unapproved indication) use of drugs and that the prescribing information provided during a satellite symposium was promotional. Another complainant claimed that Pfizer’s exhibition stalls (one of which included a stall shared with BMS in promotion of Eliquis) were extravagant and delineated a ‘party atmosphere’ rather than scientific professionalism. The Prescription Medicines Code of Practice Authority (PMCPA) investigated the cases and ruled that Boehringer Ingelheim and Pfizer were not in breach of the specified sections of the Association of the British Pharmaceutical Industry Code of Practice for the Pharmaceutical Industry.
However, the PMCPA noted that the four presentations as part of Boehringer Ingelheim’s symposium focused only on the use of dabigatran and that the final presentation included claims for a specific reversal agent for dabigatran that had not received European Union (EU) approval. The PMCPA expressed concerns that this agent may have been promoted prior to market approval. They also noted that Pfizer’s stalls had distributed coffee, tea, hot chocolate, chai latte, flavoured iced drinks and iced coffee, as well as some chocolates, which were on the ‘verge of acceptability’.46
European Haematology Association’s (EHA) 19th Congress (2014)
In June 2014, BMS sponsored 25 haematologists and Bayer sponsored one haematologist to attend the EHA 19th Congress in Milan, Italy. The sponsorship by BMS cost $A192 080 in total (on average, $A7683 per person) and included business class flight fares, accommodation, congress registration and travel for targeted delegates. BMS also sponsored a dinner for 35 haematologists attending the event, costing an additional $A4332 for one night, or $A124 per person. The sponsorship by Bayer cost $A8407 in total for one person. The event consisted of 40 presentation sessions, five of which were NOAC-related and 200 poster abstracts with eight of these NOAC-related.47–52
Posters discussed a potential partial reversal agent for apixaban, a higher incidence of ischaemic stroke and bleeding events in the real-world use of dabigatran compared with other NOACs, the favourable cost-effectiveness of the NOACs, rivaroxaban and dabigatran as advantageous and safe NOACs, less bleeding events in the NOACs compared with vitamin K antagonists, and the greater antiplatelet effect of dabigatran versus acenocoumarol. One poster was co-sponsored by Bayer, which only mentions the use of one NOAC (rivaroxaban) in patients with venous thromboembolism. Four posters had at least one author who had received speaker fees, consulting fees, research support or honoraria from at least one of the NOAC-manufacturers.
All of the speakers in the five NOAC-related sessions had previously received honoraria, study grants, speaker fees or undertaken consultation for the manufacturers of the NOACs. Presentations discussed the basic uses of the NOACs, laboratory testing of the NOACs and the use of the NOACs in venous thromboembolism, paediatric thrombosis, and cancer patients. Generally, no off-label uses of the NOACs were encouraged, however, one speaker mentioned that ‘personally, I do not think the NOACs are completely contraindicated… in cancer patients… you may choose to use a NOAC unless there is a contraindication’,51 with another mentioning that NOACs could be used in children as a ‘last resort therapy’.52 Another speaker mentioned that although more time was needed to observe the real-world use of the NOACs, ‘the NOACs are safe, if not safer, than standard care’, that there were ‘infrequent bleeding events with the NOACs’, and that the ‘NOACs have a more beneficial risk to benefit relationship compared with warfarin’.48
Discussion
Between 2011 and 2015, pharmaceutical industry-sponsored NOAC-related events aimed at Australian health professionals were frequent, with over $A10 million spent on 2797 events. These events were provided for a wide range of healthcare professionals, with almost 90 000 attendances including medication prescribers such as general practitioners, cardiologists and haematologists, as well as nurses, pharmacists and allied healthcare professionals with the potential to influence prescribing. On average, NOAC-related events had more attendees per event compared with all other events funded by the pharmaceutical industry in Australia.1
Our findings suggest that this substantial investment in NOAC-related events made by four pharmaceutical companies had a promotional purpose. Over $A4 million was spent on catering of dinners, lunches, breakfasts, teas, alcohol, and other meals and beverages for attendees. Previous studies have found that the provision of industry-sponsored meals has been associated with increased rates of brand-medication prescribing that is not always evidence based.2 3 The analysis of the two NOAC-related event case studies detected promotion of NOACs for unapproved indications and an emphasis on a favourable benefit/harm profile. Although some of the content at these events featured educational information regarding the NOACs, all speakers had financial ties with the manufacturers of the NOACs. Use of key opinion leaders is a well-documented strategy employed by industry to deliver marketing messages at events for healthcare professionals.31–33 Our findings also corroborate a previous study showing that satellite symposia tend to focus solely on the sponsor’s drug and promote unapproved uses of the drug or other similar agents.30
We observed that events began to occur before a drug was subsidised for a new indication, and that both prescribing and the number of events increased after the subsidy. A previous Australian study found that the pharmaceutical industry uses educational events to market products of low cost-effectiveness or uncertain safety in an effort to have them subsidised by the PBS.34 Our finding does not establish causality between pharmaceutical industry spending on events and increased prescribing. Other factors could also contribute to increased prescribing, such as the availability of government subsidy, increased disease incidence or awareness, and, pharmaceutical advertising. The uptake of rivaroxaban and dabigatran, in particular, may have also been aided by Product Familiarisation Programs run by the sponsors following TGA registration.7
Our study has some potential limitations. First, there was limited detail provided by the company on the content of most NOAC-related educational events. Therefore, although a list of keywords was thoroughly devised in order to filter the original dataset for NOAC-related events, some events could have been missed and our study may have under-estimated the true number of NOAC-related educational events. Second, information on the content of events was generally not publicly available, limiting our case study analysis to two major conferences. Third, we could only access data on the dispensing of the NOACs under the PBS and thus, could not account for non-PBS prescriptions for the NOACs, for example, for unapproved indications. This could have led to an under-estimation of the prescribing of the NOACs, although unsubsidised use of the NOACs is likely to be low due to their high costs. Future studies linking data on industry payments to individual-level prescribing data, similar to the investigations conducted using the Open Payments Database in the USA, could provide additional information on the association of payments and prescribing.35–39 Lastly, the transparency data are limited to Australia, although pharmaceutical companies are multinational and use similar promotion strategies around the world.40
The manufacturers of NOACs on the market in Australia have made substantial investments in sponsoring promotional events on NOACs for health professionals. These promotional activities potentially jeopardise the principles of the WHO’s Rational Use of Medicines and the Australian Government’s Quality Use of Medicines and National Medicines policies.41–43 These policies encourage healthcare professionals to provide patients with cost-effective, appropriate and safe medication. The promoted NOACs are expensive alternatives to existing therapies, and concerns about their safety have been raised. Healthcare professionals should seek independent information on NOACs from, for example, government agencies or drug bulletins. Transparency about pharmaceutical company payments should be maintained and strengthened in order to gather stronger evidence on the association of payments with prescribing.
Acknowledgments
We thank L. Parker, A. Fabbri and B. Mintzes for their contributions to building the database of disclosed payments from publicly accessible industry documents and for their feedback during the drafting of the manuscript.
References
Footnotes
Contributors LB conceived the study. BB wrote the first and subsequent drafts, extracted and analysed the data, and contributed to the study design. EAK contributed to the study design, assisted with analyses and critically revised the manuscript. LB participated in creating the original database and critically revised the manuscript. All authors reviewed and approved the final manuscript. LB affirms that the manuscript is an honest, accurate and transparent account of the study being reported and that no important aspects of the study have been omitted.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available in a public, open access repository.