Article Text
Abstract
Introduction Surgery is one of the primary treatments for lung cancer. The postoperative symptom burden experienced by patients with lung cancer is substantial, seriously delaying their recovery from surgery and impairing their quality of life. Patient-reported outcome (PRO)-based symptom management is increasingly regarded as an optimal model for patient-centred care. Currently, clinical trial-based evidence involving early-phase (immediately after surgery for up to 1 month) symptom management of lung cancer is lacking. We propose a randomised trial to evaluate the effect of a PRO-based symptom-monitoring programme with overthreshold alerts and responses for postoperative recovery in patients with lung cancer.
Methods and analysis The study will recruit 160 patients with lung cancer from six hospitals. The patients will be randomly allocated to the intervention group or control group in a ratio of 1:1. Patients in the intervention group will receive PRO-based symptom management from the specialists when their reported target symptom (pain, coughing, fatigue, disturbed sleep and shortness of breath) scores reach the preset threshold (score ≥4). Patients in the control group will not generate alerts and will follow the standard procedures for symptom management. All patients will receive symptom assessments via the MD Anderson Symptom Inventory—lung cancer module on the day before surgery, daily after surgery and twice a week after discharge until 4 weeks or the start of postoperative oncological treatment. The primary outcome—mean symptom threshold events—will be compared between the intervention and control group via independent sample Student’s t-test.
Ethics and dissemination The study was approved by the Ethics Committee of Sichuan Cancer Hospital on 22 November 2018 (No. SCCHEC-02-2018-045). This manuscript is based on V.2.0, 9 May 2019 of the protocol. The study results will be disseminated in publications in peer-reviewed journals and presentations at academic conferences.
Trials registration number ChiCTR1900020846.
- lung cancer
- patient-reported outcomes
- postoperative symptom management
- randomised controlled trial
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
This is an interventional study, comparing patient-reported outcome-based postoperative symptom management with standard postoperative symptom management in patients with lung cancer.
It is a multicentre, randomised controlled trial, conducted in six tertiary hospitals in China.
It focuses on the early postoperative period with a high frequency of data collection, including baseline before surgery, daily in-hospital after surgery and twice a week after discharge until 4 weeks or the start of postoperative oncological treatment.
We use a lung cancer-specific scale and the recall period is 24 hours, which is more suitable to measure rapidly changing symptoms during the early recovery phase.
The lack of blinding for the participants and specialists delivering the intervention may be a limitation.
Introduction
Lung cancer is the most common cancer and the leading cause of cancer death in China and worldwide.1 2 With the application of low-dose CT in screening, more and more patients with early-stage lung cancer are being diagnosed and treated with surgery. However, the postoperative symptom burden of lung cancer patients is very severe, and this detrimentally affects their quality of life (QOL). Patients have various symptoms, such as pain, coughing, fatigue and shortness of breath in the early stages after surgery or even a long time after surgery.3–5 Lowery et al followed 183 lung cancer patients for 1–6 years and found that 79.8% of them had a variety of symptoms.3 Among these patients, 30.6% had one symptom, 27.9% had two symptoms, and 21.3% had three or more symptoms. The most frequent symptoms were pain and shortness of breath. If these symptoms are not effectively controlled, the postoperative recovery of patients will be severely affected, resulting in a poor QOL.3 4 Therefore, effective interventions are needed to alleviate postsurgery symptoms in patients with lung cancer.3 4
Symptom management is the foundation of clinical care, particularly for patients with cancer. Patient-reported outcome (PRO)-based symptom management is increasingly regarded as an optimal model for patient-centred care.6 7 A PRO is a measurement of a patient's health status that comes directly from the patient's subjective evaluation, with no interpretation by medical providers or anyone else.8 Studies have shown that it may be more accurate for patients to evaluate their own health status themselves than evaluation by medical providers.9 The application of PRO-based symptom monitoring and alerting followed by real-time symptom management from healthcare professionals can improve the QOL of patients, prolong survival, increase patient satisfaction and allow evaluation of the treatment method.6 10–15 Basch et al reported a randomised controlled trial (RCT) result, suggesting that PRO-based proactive symptom monitoring could improve symptom management and thus bring survival benefits in patients undergoing chemotherapy.10 11 When compared with a traditional reactive monitoring group, median survival was 5.2 months longer among patients in the proactive monitoring group (31.2 vs 26.0 months, p=0.03).10 However, it is still not clear if adequate symptom control and improved QOL in the surgical population can ensure a potential better survival.
Currently, PROs are mainly used in non-surgical treatment settings,10–13 and they are still in the early stage of application in the surgical treatment setting.5 7 14–28 Studies on postoperative symptom management of lung cancer, especially in the early postoperative period, are lacking. In addition, most of the published literature has a low level of evidence due to its design.5 18–28 The limitations of these studies include: (1) most were observational studies; (2) they had small sample sizes ranging from 30 to 200 subjects with few exceptions; (3) they did not focus on the early postoperative period, typically including the in-hospital period immediately after surgery and 4 weeks after discharge when patients frequently report multiple severe symptoms, leading to later negative recovery events, that is, higher symptom burden, delayed return to intended oncological therapy and poorer QOL; (4) they used a variety of survey instruments and some were not a lung cancer-specific scale; (5) most of the scales used, such as the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–Lung Cancer Module, had a recall period of 1 week, which may not be able to identify the rapidly changing symptoms during early postoperative phase5; (6) only one study assessed in-hospital patients immediately after surgery, but the MD Anderson Symptom Inventory (MDASI) scale used did not include lung cancer-specific symptoms, that is, coughing5; (7) the symptom assessments were inadequate, mostly at just two or three time points postsurgery; (8) most of the surgical approaches were thoracotomies, not representing the current mainstream minimally invasive thoracoscopic surgery for lung cancer; (9) there were very few studies on the Chinese population.
We have been conducting an observational study of perioperative symptom in patients with lung cancer based on PRO (registration number NCT03341377). Now, we propose an RCT, aiming to evaluate the efficacy of a PRO-based symptom monitoring, alerting and response system (SMARS) to improve postoperative recovery of lung cancer patients. The SMARS (figure 1) includes a research electronic data capture (REDCap) platform, an electronic PRO system (ePRO Hub) and a most popular social software (WeChat)29 in China. This study will provide evidence for early postoperative phase symptom management for patients with lung cancer. We will use the MDASI lung cancer-specific scale (MDASI-LC)30 to frequently monitor symptoms and their impact on the functioning of patients with lung cancer from preoperation to 4 weeks after discharge or until the beginning of postoperative oncological treatment. The recall period of MDASI-LC is 24 hours, which is more suitable to measure rapidly changing, early postoperative period symptoms compared with other QOL scales for 1 week or longer. Our research hypothesis is that patients with lung cancer undergoing PRO-based symptom management have a lower postoperative symptom burden than patients undergoing standard symptom management.
Schematic diagram of the SMARS. ePRO, electronic patient-reported outcome; REDCap, research electronic data capture; SMARS, symptom monitoring, alerting and response system.
Methods and analysis
Study design
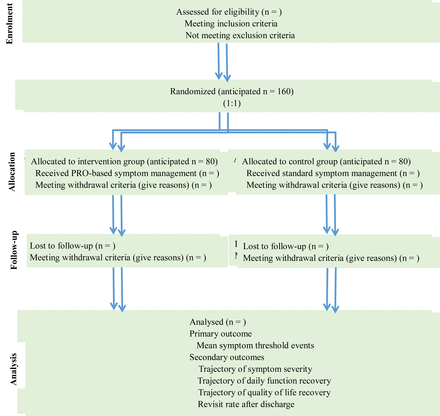
The trial is a multicentre, randomised, parallel group controlled and superiority design. This protocol will be consistent with the Standard Protocol Items: Recommendations for Interventional Trials.31 The results of this trial will be reported according to the guidelines of Consolidated Standards of Reporting Trials.32 A flow chart of this trial is shown in figure 2.
Flow chart of this parallel group randomised trial. PRO, patient-reported outcome.
Setting
Participants will be recruited from six tertiary hospitals in different cities in China. The six hospitals are Sichuan Cancer Hospital, Zigong First People’s Hospital, Jiangyou People’s Hospital, Dazhu County People’s Hospital, The Third People’s Hospital of Chengdu and The Seventh People’s Hospital of Chengdu. The total number of lung cancer operations in six hospitals is approximately 2000 per year.
Participant recruitment
Participant recruitment will be carried out before the surgery by participating clinicians. Eligible patients should meet all the inclusion criteria and not meet any of the exclusion criteria. The inclusion criteria for the participants are: (1) aged 18 to 75 years, (2) clinically diagnosed as primary lung cancer, (3) clinical stage I-IIIA (eighth edition)33 (4) planning to receive surgery and (5) able and willing to respond to a repeated electronic questionnaire (e-questionnaire) on a smartphone or a tablet. The exclusion criteria are: (1) history of neoadjuvant therapy, (2) having other malignant tumours and (3) unable to understand the study requirements.
Strategies for achieving adequate participant enrolment to reach a target sample size include inviting more doctors in each centre to participate in the study and adding more research centres. Plans to promote participant retention and complete follow-up include education, refill reminders and commitments to provide all the patients with free long-term medical consultations after the trial via WeChat. In China, the first follow-up clinic visit of surgical lung cancer patient is approximately 4 weeks after discharge. There is no usual follow-up within these 4 weeks. In addition, usual care does not include free medical consultations after discharge. Patients usually have to pay for follow-up care. Free long-term medical consultation is an incentive for patients who participate in the study, which may improve compliance. This incentive will do more good than harm to the patients, so it is approved and recommended by the Ethics Committee of Sichuan Cancer Hospital. The anticipated dates of the study are from 1 December 2018 to 31 December 2020. We haven't started recruiting patients yet.
Sample size calculation
The primary end point of this study is the mean symptom threshold events, defined as the average number of target symptom threshold events per patient, at each time point. To meet the minimal clinically important difference (0.5 SD)34 for the mean symptom threshold events, the required sample size is 64 for each group, when rejecting the null hypothesis (the difference between the two groups <0.5 SD). A total of 128 cases with valid data are needed. Considering a 20% attrition rate, we will need 80 patients for each group (64/0.8). The 20% attrition rate is based on our ongoing observational research (NCT03341377). The current withdrawal rate is about 17% in the observational research. The rate of loss to follow-up in this trial is estimated to be less than 3%, because this trial is an interventional study and the follow-up time is very short (less than 4 weeks). In this trial, the 20% attrition include both withdrawal and loss to follow-up. The sample size calculation is based on the independent sample Student’s t-test, using a two-tailed alpha level of 0.05 and a beta error probability of 0.02 (80% power).
Randomisation and allocation concealment
The process of randomisation will be carried out online using the central randomisation module on the REDCap platform (http://125.71.214.100:888/redcap) after a participant has been recruited to the study and has signed an informed consent form. The data manager will upload the randomisation allocation table to the REDCap platform and then save the randomisation allocation table independently. The investigator will conduct randomisation by clicking on a randomisation button on the REDCap platform. It will then be allocated to the intervention group or control group with a 1:1 ratio. Each group will have 80 cases.
Blinding
The blinding of participants and specialists delivering the intervention is impossible due to the nature of the interventions. But the data collectors who help administer PRO collection will be blinded to group allocation to minimise measurement bias. The statisticians analysing the results will also be blinded to group allocation.
Intervention
After enrolment, all the patients will use their WeChat app to connect with the participating specialists’ WeChat app via a mini programme (ePRO Cell). Then, they will be taught how to use the programme. The ePRO questionnaires will be set to send to the patients’ WeChat app automatically after randomisation. Patients are required to complete the ePRO questionnaires on their smartphones or tablets before surgery (baseline, typically 1–3 days before the operation), daily after surgery (in-hospital, typically 1 to 7 days after the operation) and twice a week after discharge until 4 weeks or the start of postoperative oncological treatment (typically collecting PRO data six to eight times after discharge). In a hospital setting, if the patients do not complete the ePRO questionnaires within the scheduled time, an electronic reminder (e-reminder) and up to two bedside reminders will be delivered at the same day. After discharge, if the patients fail to complete the ePRO questionnaires within the scheduled time, an e-reminder and up to two phone reminders will be delivered with 24 hours.
Comparison
Intervention group
Patients will not be informed about the threshold levels. When there are one or more target symptoms (pain, coughing, fatigue, disturbed sleep and shortness of breath) and scores reach the preset intervention threshold (score ≥4), the participating specialist (thoracic surgeon) will simultaneously receive an alert message on his or her WeChat. The specialist will manage the patients’ symptoms based on the scores of the PRO. After discharge, the specialist will mainly use the WeChat or sometimes a telephone to contact the patient within 24 hours to implement symptom relief measures, for example, consultation, education, medication guidance and clinic or hospital visit suggestions. The symptom relief measures of the intervention group patients will comply with the latest guidelines and be standardised across all centres, in the form of a standard operating procedure (SOP) handbook. Patients’ adherence to the interventions will be asked at each time point. Those who do not follow the specialist’s advice will be monitored, and the number of violations will be recorded. Those who refuse to follow the specialist’s advice more than three times will be considered as seriously violating the study protocol and will be withdrawn. Patients will be educated and allowed to seek medical help through usual channels for severe symptoms.
Control group
The control group patients will be informed that the ePRO data collected are only for scientific research. They will not generate any alerts or get responses relating to their symptoms. The patients’ symptom management will follow the current standard postoperative management model. During hospitalisation, the doctors manage the control group patients’ symptoms based on their own judgement rather than the scores of the PRO. After discharge, the patients will go home and the first clinic visit is approximately 4 weeks later. Patients will be encouraged to seek medical help if severe symptoms are reported.
Withdrawal criteria
Participants will be withdrawn from the study, and no further data will be collected if they meet the following criteria: (1) unexpected cancellation of surgery, (2) severe postoperative complications (≥grade Шb according to the Clavien–Dindo classification of surgical complications) affecting symptom data collection, (3) postoperative length of stay >14 days (because patient with a postoperative hospital stay >14 days usually has a severe complication, and the patient compliance will gradually decrease, affecting the accuracy of PRO data), (4) postoperative pathology shows non-primary lung cancer, (5) non-R0 resection, (6) pathological stage IV, (7) participant seriously violates the study protocol (continually not complying with the specialist’s advice, intentionally letting a proxy to complete the PRO surveys and deliberately providing false PROs) or (8) participant asks to withdraw from the study.
Outcomes and measurement
Primary outcome
The primary end point of this study is the mean symptom threshold events. According to our pilot study, the five most common postoperative symptoms of patients with lung cancer are: pain, coughing, fatigue, disturbed sleep and shortness of breath. In this study, these five symptoms assessed by the MDASI-LC are defined as target symptoms. According to the recommendation of National Comprehensive Cancer Network and published literature, when a patient’s symptom score is ≥4, it is identified as moderate severity.35 36 In this study, a score of 4 is set as the threshold value for intervention, and a target symptom score of ≥4 is reported as a threshold event.
The primary PRO tool used in this study is the MDASI-LC.30 It is a measure that contains 16 items of lung cancer-related and treatment-related symptoms and six items of interference to normal daily life caused by symptoms. All items are rated on 0–10 numerical scales, with 0 representing ‘symptom not present’ or ‘symptom not interfered with life’ and 10 representing ‘symptom as bad as one can imagine’ or ‘symptom completely interfered with life’. The recall period of the MDASI-LC is 24 hours, and it can usually be conducted in 5 min. It has been translated and validated for application in a Chinese context.
Secondary outcomes
The secondary end points of this study include trajectories of PROs (symptom severity, daily functioning and QOL) and revisit rate after discharge. Trajectories of PROs are defined as the longitudinal changing pattern of the mean score of the five target symptoms for symptom severity, the mean score of the six MDASI-LC interference items for daily functioning and the mean score of the single-item QOL scale (UNISCALE) for QOL37 from the baseline to 4 weeks after discharge or until the start of postoperative oncological treatment. UNISCALE has only one question using a 0–10 scale, with 0 representing ‘worst QOL’ and 10 representing ‘best QOL’. The revisit rate after discharge is defined as the ratio of the number of patients who see the doctor again after discharge including outpatient visits, emergency visits and hospitalisation divided by the total number of patients.
Other data
The clinician workload, clinician system acceptability and patient satisfaction of the interventions will be assessed through surveys and interviews. Demographics, clinicopathological characteristics, follow-up information and adverse events of the interventions will also be collected. All the adverse events will be assessed and managed by a thoracic surgeon.
Data collection, management and quality control
REDCap,38 39 a worldwide popular research data collection and management platform established in Sichuan Cancer Hospital, will be used for data collection and management in this study. PRO data will be collected using e-questionnaires and recorded in REDCap. Participants should fill out the e-questionnaires by themselves. If participants have difficulties in completing the e-questionnaires, data collectors or their family members will help them by just reading each item aloud and recording the participant’s responses. The control group patients’ PRO data will not be accessed by the specialists. Specialists can only access the PRO data of the intervention group patients. Other data including demographics, clinicopathological characteristics and follow-up information will also be entered into the REDCap database.
Data will be checked regularly by the quality controller. Participant privacy information will not be recorded in REDCap. A study number will be allocated to each participant and will be used on all study documentation, which will only be available to the investigators. Before patients’ enrolment, investigators from each research centre will receive SOP training. Each centre will receive on-site monitoring visits, telephone monitoring and online guidance during the course of the trial.
Data analysis
Per-protocol analyses will be conducted. To be included in the analysis, a participant must provide MDASI-LC data from the baseline and at least two additional time points. If a participant meets the withdrawal criteria, no data will be included in the analysis. Two-sided p values of <0.05 are considered to be statistically significant. Continuous variables will be presented as mean±SD or median and IQR. Comparisons between groups will be conducted using the Student’s t-test or the Wilcoxon rank sum test. Categorical variables will be presented as frequencies or proportions and compared between groups using the χ2 test. Trajectories of PROs will be compared between the intervention group and control group using generalised mixed effects models. Missing data will be processed by the multiple imputation method. Results obtained from data without missed observations will be compared with that from imputed data for sensitive analysis.
Data monitoring and interim analysis
A data monitoring committee (DSM) consisting of one clinician, one statistician and the secretary of the Ethics Committee of Sichuan Cancer Hospital will be set up. Study monitoring will be carried out regularly by DSM members, and the process will be independent from investigators. Due to the low risk of the study content and short-term study duration, interim analysis will not be performed.
Patient and public involvement statement
Patients and the public will not be involved in the design, recruitment to or conduct of this study. We will inform the applicants of the results. There are no plans to disseminate the results to study participants, because it is not a routine practice to feed back research results to participants in China. Participants will be informed that they can obtain the final results of this study through our future published articles.
Ethics and dissemination
All recruited patients will be required to give written informed consent. Any subsequent amendments to the protocol will be submitted for further review and approval. Subcentres will gain approval from their hospital-specific ethics committees. The results of this study will be disseminated through peer-reviewed publications and academic conferences.
Discussion
This trial focuses on the early-phase postoperative symptom management after lung cancer surgery. The potential implications of the findings include: (1) identifying if PRO-based symptom management is better than usual symptom management, (2) identifying if proactive symptom management can reduce symptom burden and improve QOL in the surgical population, (3) laying a foundation for future research on whether postoperative symptom management improves survival, (4) investigating whether SMARS is feasible and acceptable in real-world clinical practice in China and (5) identifying barriers which will be used to facilitate further revisions of the SMARS and help extend its implementation in non-surgical settings.
There are many limitations in this trial. First, the trial will be carried out in well-resourced tertiary hospitals in China. This will limit the generalisability to non-tertiary hospitals. Second, the inclusion criteria and exclusion criteria are strict. For example, the programme is unsuitable for patients without internet access or with poor literacy. This will greatly limit the population for which this study is applicable. Third, the withdrawal criteria will create selection bias and limit the external validity, although the strict criteria will ensure the compliance of this study. In the future, we will conduct pragmatic clinical trials (PCT) to evaluate the effectiveness of the monitoring system in a more heterogeneous population to improve the generalisability. Fourth, the lack of blinding for the participants and specialists delivering the intervention will also be a limitation, because it may increase the measurement bias. Fifth, it may affect the establishment of feasibility if patients are not involved in the design and development of this trial, although previous studies and our ongoing observational study have provided pilot data for the design and development of this trial in terms of feasibility and acceptability. This RCT is designed to test the efficacy of the PRO monitoring system. We will evaluate the effectiveness in a future PCT, with patients’ involvement in study design, conduct and interpretation. Sixth, the follow-up period is very short. The results need confirmation in a study with a longer follow-up period.
In summary, as a RCT, this study will test the efficacy of SMARS in postoperative care and provide data of feasibility for further unblinded pragmatic study when implementing the SMARS in the real world, with the involvement of community hospitals and patients with poor socioeconomic status, while a wider internet access is available for the whole Chinese population.
Acknowledgments
The authors thank all the patients and patient advisers who are involved in this study.
References
Footnotes
WD and YZ contributed equally.
Contributors WD and QS conceived and designed the study. WD and QS obtained the funding. WD is the chief investigator of this study. YZ, WF, XL, YM and RZ are subcentre principal investigators who contributed to the trial feasibility stage. WD, YZ, WF, XL, YM, RZ, XW, CW and SX drafted the protocol. QS participated in the statistical plan. QL and QS revised the manuscript. All authors have read and approved the manuscript. All authors have met the ICMJE criteria for authorship.
Funding This work was supported by Bethune charitable foundation, Sichuan Science and Technology Program (grant number: 18PJ436 and 2019YFH0070) and National Natural Science Foundation of China (grant number: 81872506).
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval This study was approved by the Ethics Committee of Sichuan Cancer Hospital on 22 November 2018 (No. SCCHEC-02-2018-045).
Provenance and peer review Not commissioned; externally peer reviewed.