Article Text
Abstract
Objective The limited existing asthma control questionnaires that are available for children 5 years of age or younger in China mostly assess only the impairment domain of asthma control. Here, the English version of the Test for Respiratory and Asthma Control in Kids (TRACK) was translated into Chinese and validated for its application in asthma control in preschool children.
Design Prospective validation study.
Setting and participants A total of 321 Chinese preschool children suffering from asthma completed the study from December 2017 to February 2018.
Method The TRACK translation into Chinese employed the translation and back translation technique. The caregivers of the preschool children with asthma symptoms completed TRACK during two clinical visits over 4–6 weeks. Moreover, the physicians completed a Global Initiative for Asthma (GINA)-based asthma control survey at both visits. The utility of TRACK for assessing the change in asthma control status and its reliability and discriminant validity were evaluated.
Results The Chinese version of TRACK showed internal consistency reliability values of 0.63 and 0.71 at each visit, respectively (Cronbach’s α). The test–retest reliability was 0.62 for individuals whose GINA-based assessment results were the same at both visits (n=206). The TRACK scores for the children in the various asthma control categories were significantly different (p<0.001). Children recommended for increased treatment by the physicians had lower TRACK scores than those recommended for no change in treatment or decreased treatment (p<0.001).
Conclusion The study verifies the validity and reliability of the Chinese version of TRACK. Changes in the TRACK scores effectively reflected the level of asthma control in preschool children and guided further treatment strategies.
Trial registration number NCT02649803
- asthma
- asthma control
- preschool children
- questionnaire
- reliability
- validity
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
The present study was the first to validate the Chinese version of the Test for Respiratory and Asthma Control in Kids for preschool children with asthma.
The study sample was recruited from the Yangtze River Delta region, represented by Jiangsu, Zhejiang and Shanghai, where the incidence of asthma in children has increased rapidly over the last 10 years.
Only children 5 years of age or younger with asthma were included, and patients with other recurrent wheezing diseases were excluded.
The main limitation of this study was that the caregivers had relatively high educational backgrounds, which may limit the surveys applicability to other underdeveloped provinces in China.
Introduction
Since 1990, the prevalence of asthma in paediatric patients has remarkably increased in China. The prevalence in children aged 0–14 years was 1.07% in 1990, 1.97% in 2000 and 3.02% in 2010, resulting in a major public health problem. The prevalence of asthma has been increasing steadily since 1998, and the prevalence of children with asthma has likely increased from 2017 to 2019.1 Preschool children (those aged 5 years or younger) present significantly higher morbidity from asthma than those in other age groups. In addition, there were 4.27 exacerbations per 10 person-years in preschool children in a population-based cohort study.2 The annual rate of emergency department visits and hospital admissions is higher than that of other age groups.3 Preschool childhood wheezing may reflect a progressive decline in lung function that could extend into adulthood and an elevated risk of chronic obstructive pulmonary disease (COPD) when accompanied by atopy.4 Asthma management in preschool children is complex, as the effects of different therapies for varied phenotypes remain unclear, and several confounders can affect the treatment response. As a result, preschool children with asthma require a large amount of healthcare resources, resulting in a high economic burden.
Poor treatment adherence represents a significant risk factor in children with asthma.5 Because of the lack of an effective caregiver-reported asthma control assessment tool for preschool children, caregivers usually underestimate the child’s asthma symptoms; this is one of the primary reasons for poor treatment compliance.6 Evaluation of the asthma control level in children with asthma remains an essential factor in the follow-up and treatment of this chronic disease. Current guidelines emphasise the assessment of asthma control, including clinical asthmatic manifestation assessments and lung function screening.7 Preschool-aged children are too young to complete a lung function test; therefore, asthma control assessments of these individuals are mostly dependent on caregiver feedback. Thus, assessing the control level in these individuals remains challenging. Over the past few years, many questionnaires have been proposed to evaluate asthma control in children aged 4–11 years8 and 5–17 years9, as well as in adolescents and adults.10 The Global Initiative for Asthma (GINA) and the National Asthma Education and Prevention Programme (NAEPP) have emphasised two asthma control domains: risk and impairment. However, most existing asthma control questionnaires cannot be used for children under 5 years of age and assess only the frequency of respiratory symptoms and rescue drug usage.11
A simple, efficient and validated tool is urgently needed for preschool children with asthma in China. In 2007, Murphy et al developed a new assessment tool called the ‘Test for Respiratory and Asthma Control in Kids (TRACK)’ for children 5 years of age and younger, covering the risk and impairment domains. This caregiver-reported questionnaire contains five items. Each item is assigned a score of 0–20 points based on a 5-point Likert scale for a total of 0–100 points. The reliability of TRACK was >0.7 in the development and validation samples. While screening for control issues, TRACK displayed a good area under the receiver operating characteristic (ROC) curve based on the NAEPP-based evaluation of asthma control. TRACK correctly classified asthma control levels in approximately 80% of preschool-age individuals with asthma, and the cut-off point was 80.12 TRACK score alterations ≥10 points are clinically significant for respiratory control in young children showing respiratory symptoms indicating asthma and should trigger a re-evaluation of asthma management.13 However, the questionnaire has not been validated in China.
The current study aimed to propose and validate a Chinese version of TRACK to evaluate asthma control in preschool children. This questionnaire can be used as a complement to the limited asthma control assessment tools that are currently available in China for children 5 years of age or younger with asthma.
Methods
All caregivers provided signed informed consent before study initiation. The trial is registered as NCT02649803 on ClinicalTrials.gov. The study protocol has been published in BMJ Open.14
Study design and setting
The current prospective, multicentre, observational trial was carried out from December 2017 to February 2018 at Shanghai General Hospital (Shanghai), Shanghai Children’s Medical Center (Shanghai), Nanjing Children’s Hospital (Nanjing), The Children’s Hospital (Hangzhou) and 14 community hospitals in Pudong District, Shanghai. The staff of all the community hospitals contributing to the present trial received systematic training prior to patient enrolment.
Study population
The caregivers of the preschool children with asthma in the ‘paediatric asthma control under a community management model in China’ clinical study programme who were invited and visited the study site to participate were given a concise description of the trial.14 The inclusion criteria were as follows: (1) the child was an outpatient ≤5 years of age and of either sex; (2) the child received a diagnosis of asthma based on the GINA criteria (a history of three or more times of wheezing attack per year in the absence of obvious respiratory infection; exercise-induced, laughing-induced or crying-induced wheezing or coughing; clinical improvement with 2–3 months of regular low-dose inhaled corticosteroids (ICSs) and symptom worsening after ICS cessation); (3) the child’s parent or guardian provided consent and (4) the caregiver had access to a smartphone. The exclusion criteria were as follows: (1) the child had congenital heart disease, gastro-oesophageal reflux, bronchopulmonary dysplasia or bronchiolitis obliterans; (2) the child had a previous allergic reaction to an ICS; (3) the child presented other ailments that could potentially interfere with the study data according to the physician and (4) the child was involved in a similar trial in the past 3 months.
Atopic dermatitis was diagnosed by a senior dermatologist by examining the skin and reviewing the child’s medical records. The diagnosis of allergy rhinitis was established by a senior ear, nose and throat consultant according to Allergic Rhinitis and its Impact on Asthma guidelines.15 A food allergy was diagnosed by an allergist-immunologist based on a number of factors, such as symptoms, family history, skin and blood tests, elimination tests and oral food challenge.
The sample size in validation studies should exceed 5–10 times the number of parameters.16 Tabachnik and Fidell proposed that ≥300 cases were required for a factor assessment.17 These recommendations were used for the sample size determination.
The caregivers were trained to use the study application (APP) installed on their smartphones. We monitored whether the caregivers completed the TRACK report and reminded the users to complete the report every month to ensure compliance. The caregivers completed the TRACK report on their smartphone before they entered the consultation room. The caregivers were able to read and write in Chinese.
TRACK questionnaire
The caregiver-reported TRACK contained five items to monitor respiratory control in children 5 years of age or younger. TRACK included the frequency of respiratory manifestations (such as wheezing, coughing and shortness of breath), night-time awakenings, activity limitations in the last 4 weeks, the frequency of rescue medicine utilisation in the preceding 3 months and oral corticosteroid administration in the past 12 months. Scores for various items ranged between 0 and 20, and the total score of the TRACK questionnaire was 100.12
The English version of TRACK was translated into Chinese following previously established guidelines.18 First, a forward translation was carried out independently by two native Chinese-speaking investigators with English fluency who were paediatricians with a public health background to produce a consensus version. Second, the consensus version was back-translated into English by two blinded professional translators. Finally, the original, translated and back-translated versions were thoroughly compared by a committee of experts for conceptual equivalence. Then, a prefinal consensus version was obtained.
Although the Chinese version of TRACK attempted to maintain consistency with the original version of the questionnaire, its content was partially adjusted. The modified Chinese version of TRACK was slightly revised for item 5 of the prefinal consensus version after communicating with Professor Murphy, the original author of TRACK. The following question was added as item 5: “How often does your child take a high dose of ICSs (nebulised budesonide 1 mg/dose, daily inhalations or other equivalent ICSs) and systemic corticosteroids (oral prednisone, oral prednisolone, intravenous methylprednisolone, intravenous hydrocortisone succinate) for breathing issues when not controlled by other medications?” The treatment of asthma in China is more likely to involve the use of high-dose ICSs to reduce or avoid the use of systemic corticosteroids based on the GINA data.7 In addition, some children who experience severe asthma attacks are prescribed intravenous corticosteroids (IVCS) according to the GINA assessment7 during emergency treatment hospitals in China. Therefore, we added intravenous corticosteroid use to item 5 as a complement. Thus, the modified Chinese version of TRACK was considered to be more suitable for children aged 5 years or younger with asthma in China.19 A final Chinese version was obtained after pretesting the prefinal version on the caregivers of 10 patients; the pretests were followed by interviews to ensure comprehension and applicability to the patient population. At this point, the final version was prepared for validation. The 10 caregivers participating in the pretest did not participate in the study itself. All steps followed the TRACK’s copyright requirements.12 The Chinese version of TRACK is presented in table 1.
Chinese version of TRACK
Data collection
After informed consent was obtained, the caregivers were initially prompted to complete TRACK by using the APP on their smartphones, with a follow-up after 4–6 weeks. The physicians were blinded to the caregivers’ responses to TRACK. The asthma control levels of the patients were evaluated by physicians based on the GINA assessment for children under 5 years of age; GINA assessed four items: the frequency of daytime symptoms, nocturnal symptoms, rescue drug (bronchodilator) usage and the limitation of daily activities in the past 4 weeks. According to the GINA results, the patients were divided into three groups, including the controlled, partly controlled and uncontrolled groups.7
Patient and public involvement statement
No patients or the public were involved in the present study design. The caregivers were involved in the study by actively completing the questionnaires on their smartphones during the 2-month study period. A results report was sent to the study participants.
Statistical analysis
SPSS V.21.0 (IBM SPSS Statistics, USA) was employed for the statistical analyses. Descriptive statistics were performed for the general participant features. The Kolmogorov-Smirnov test was used to examine the normality of the data distribution. Medians and quartiles were adopted to describe non-normally distributed data. The group difference was calculated using the Kruskal-Wallis test, as the data had a skewed distribution; p<0.05 indicated statistical significance.
Reliability
Cronbach’s α coefficient served as a metric for assessing the reliability of the scale. A test–retest analysis was performed to evaluate the temporal stability of TRACK, that is, the reliability of identical responses at the first (test) and final visit 4–6 weeks later (retest). The test–retest reliability of the TRACK questionnaire was evaluated using Pearson’s correlation coefficient by comparing the scores at baseline and at follow-up in individuals whose physicians indicated that the asthma control status according to the GINA assessment was unchanged between the two visits.
Validity
Construct validity is commonly employed to assess the efficiency of a test to measure the intended outcome. An exploratory factor analysis produces the dimension of differentiation that is used to confirm the questionnaire construct validity. To determine whether the questionnaire was suitable for factor analysis, the following methods were used. The first was the Kaiser-Meyer-Olkin Measure of Sampling Adequacy (KMO) criterion, which assesses sample sufficiency, and the other was Bartlett’s test of sphericity, which examines whether questionnaire items are interindependent. Generally, a KMO value >0.6 and Bartlett’s test of sphericity at p<0.05 indicate the factorability of a correlation matrix. An exploratory factor analysis was then performed with five items by principal component analysis extraction and varimax rotation, with a minimum factor loading cut-off point of 0.4. Construct validity was analysed among the children with asthma at baseline. For the discriminant validation tests, the children were divided according to their differences in respiratory control based on two criteria. The first part of the TRACK’s discriminant validation was assessed by comparing the TRACK scores of the three categories based on the GINA definition of control (controlled, partly controlled and uncontrolled). The second part of the TRACK’s discriminant validation was assessed by comparing the TRACK scores of the three categories of treatment decisions at the end of the visit (increased therapy, no change, decreased therapy).
Screening accuracy
The accuracy of TRACK for identifying individuals presenting respiratory control issues (according to the GINA assessment) was assessed by ROC curve analysis. The children were grouped into two groups, the not well-controlled group (partly controlled and uncontrolled) and the controlled group, to detect children with any uncontrolled symptoms of asthma as much as possible. In addition, the sensitivity, specificity, positive predictive value and negative predictive value, false-positive rate, accuracy and the area under the ROC curve were calculated to explore the optimal cut-off point for screening.
Results
Demographics
Only seven patients who met the eligibility criteria were unwilling to participate in the trial. A total of 340 caregivers were recruited for the study. Of these, 321 (94.4%) caregivers completed the follow-up visit, and their TRACK reports were finally evaluated (figure 1). Most of the caregivers were female (84.1%), aged 25–44 years (81.3%) and had graduated from college or obtained a relatively high level of education (77.9%). The patients’ age distribution was as follows: <24 months, 40 (12.5%); between 25 and 48 months, 165 (51.4%) and between 49 and 60 months, 116 (36.1%). A total of 90% of the participants were reported to have a controlled asthma status according to their caregivers. Table 2 summarises the baseline characteristics of the patients and their caregivers.
Caregiver and patient demographic characteristics
Flow diagram for the selection of participants.
Reliability
The internal consistency reliability values (Cronbach’s α) were 0.63 and 0.71 at baseline and follow-up, respectively. After deletion of item 5 (oral corticosteroid (OCS), IVCS or high-dose ICS utilisation in the last 12 months), the Cronbach’s α values increased to 0.73 and 0.75 at baseline and follow-up, respectively. At baseline, Cronbach’s α was less than the value for a multi-item scale (0.7) and negatively influenced by item 5 of TRACK. The intraclass correlation for test–retest reliability was 0.63 (95% CI 0.52 to 0.73, Pearson’s correlation) for the preschool children with asthma whose physician evaluations according to GINA were the same at both visits (n=206).
Construct validation
The KMO values were 0.75 at the baseline visit and were considered satisfactory (>0.6), suggesting that the sample size was sufficiently large for assessing the factor structure. In Bartlett’s test, a χ2=350.88 (p<0.001) was obtained. Moreover, the KMO values for various constructs exceeded 0.6 with Bartlett’s test showing significance, suggesting a sufficient amount of data for factor analysis. The exploratory factor analysis was then conducted. The items of the Chinese version of TRACK showed loading on the same factors. The five items explained 52.51% of the variance. The factor loading of each item of TRACK ranged from 0.48 to 0.83 (table 3).
Loadings of the track
Discriminant validation
The TRACK scores were significantly different among the controlled, partly controlled and uncontrolled groups as categorised according to GINA, which was evaluated by the physicians at baseline (p<0.001) and follow-up (p<0.001) visits to support the discriminant validity of the TRACK scores. The TRACK scores showed the highest and lowest values in patients with controlled and uncontrolled ratings (table 4). Children who were recommended for stepped-up therapy showed significantly lower TRACK scores at baseline and follow-up than those who were recommended for no therapy change or stepped-down therapy (p<0.001, table 5).
TRACK scores based on the control levels of asthma as assessed by the GINA survey
TRACK scores based on the physicians’ recommendations according to GINA-based control
Screening accuracy
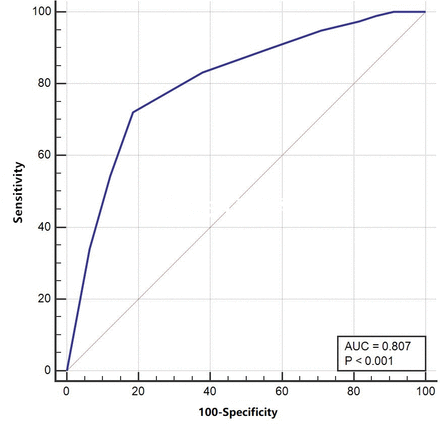
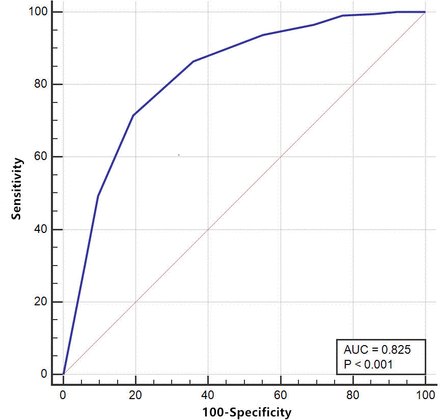
Baseline and follow-up TRACK scores (0–100) produced areas under the ROC curve values of 0.81 (figure 2) and 0.83 (figure 3) for screening ability, respectively. To distinguish ‘controlled’ patients from ‘partly controlled’ and ‘uncontrolled’ patients, a TRACK cut-off value of 85 was considered for baseline (sensitivity, 81.4%; specificity, 72.1%) and follow-up visits (sensitivity, 80.7%; specificity, 71.5%). The screening accuracies of the TRACK scores at various cut-off points at baseline and follow-up visits are presented in tables 6 and 7.
The screening accuracy of the TRACK scores at the baseline visit
The screening accuracy of the TRACK scores at the follow-up visit
Receiver operating characteristic (ROC) curve for the baseline Test for Respiratory and Asthma Control in Kids (TRACK) scores. AUC, area under the curve.
Receiver operating characteristic (ROC) curve for the follow-up Test for Respiratory and Asthma Control in Kids (TRACK) scores. AUC, area under the curve.
Discussion
To the best of our knowledge, the present study was the first to validate the Chinese version of TRACK in children 5 years of age or younger with asthma in China. The results showed that TRACK had good reliability and validity, and the responsiveness to asthma control alterations over time indicated the utility of the questionnaire.
In this study, Cronbach’s α values for TRACK were 0.63 and 0.71 at both visits, respectively. In the original version of TRACK, Cronbach’s α values ranged between 0.71 and 0.75.12 In the Spanish and Turkish versions of TRACK, Cronbach’s α values ranged from 0.74 to 0.76 in the questionnaire.20 21 In comparison with the above versions of TRACK, the Chinese version had a similar and acceptable reliability. The five TRACK items conformed to the NAEPP asthma management guidelines for both the impairment and risk domains of control assessment. The above findings indicated that TRACK confirmed asthma control to be multidimensional. However, after the deletion of item 5 (OCS, IVCS or high-dose ICS utilisation in the preceding 12 months), the internal consistency reliability values increased to 0.73 and 0.75 at baseline and follow-up in this study, respectively. The risk domain assessment demonstrated that recent severe asthma exacerbation is an important independent predictor of future severe exacerbations in paediatric patients suffering from severe or difficult-to-treat asthma and should be taken into consideration in asthma management plans.22 23 The test–retest reliability was ‘good’ in this work, but it was not ‘excellent’. A total of 4–6 weeks separated the baseline and follow-up visits to allow the evaluation of asthma control changes. Because clinical respiratory symptoms in preschool children with asthma change frequently, 4–6 weeks may not be an optimal time interval to evaluate the test–retest reliability, which could ultimately affect the results.
Another common method for assessing asthma control in preschool children is the GINA assessment, which is widely accepted and used in China and is administered by physicians. Discriminant validity was evaluated by the differences in TRACK among children with controlled, partly controlled and uncontrolled asthma based on the GINA assessment and the children whose baseline visits prompted a stepped-up, stepped-down or non-changed therapy. Our findings regarding the TRACK’s discriminant validity agreed with those of Chipps et al.24 The study recruited 438 caregivers of children with asthma below 5 years of age for TRACK completion at two clinical visits. Moreover, physicians completed the guidelines-based respiratory control survey and decided whether therapy should be changed. The results showed that the mean TRACK scores were markedly different among the children grouped by the physicians’ NAEPP-based control rating at baseline and follow-up, suggesting a change in therapy and control status and supporting the discriminant validity of the TRACK scores. These studies expanded the TRACK’s validity and reliability by demonstrating that it responded to changes in the respiratory control status of individuals with asthma under 5 years of age. Taken together, these results showed that TRACK has good validity, consistent with other versions.
However, asthma control assessment tools should be based on objective quantitative evaluations and differentiate the control levels. The optimal asthma control assessment tool quantifies asthma control as a continuous variable and provides a numeric value to distinguish between controlled and uncontrolled asthma. If the physician or caregiver knows the specific score, they will have a clearer understanding of asthma control, and it will facilitate comparisons between different periods. Therefore, we need an objectively quantified assessment tool to assess the control level of asthma in children. These requirements were met by the TRACK assessment, and we therefore consider it a complementary assessment tool to the GINA assessment for children under 5 years of age.
In the pioneering work by Murphy et al, a cut-off point of 80 yielded the best balance between sensitivity and specificity for discriminating between controlled and uncontrolled asthma cases. In patients with the TRACK scores below 80, a subsequent evaluation or treatment adjustment should be considered.12 Other versions of TRACK also used 80 as the cut-off point.20 21 Here, a cut-off of 85 yielded acceptable screening statistics at both visits. The elevated value in our study was likely because of our ROC curve that was relative to the GINA-based ratings of asthma control rather than the NAEPP-based ratings used in the other studies.12 20 21 Kaya et al evaluated the consistency between the TRACK scores and asthma control levels based on the GINA and NAEPP guidelines in preschool-aged children. With 80 as the cut-off point for TRACK, the compatibility rate of asthma control levels between the TRACK and GINA assessments was 71.0%, while that between the TRACK and NAEPP assessments was 76.4%. The non-conformity rate of the GINA results was higher than that of the NAEPP results.25 The main difference between the GINA and NAEPP guidelines is that if the daytime symptoms occurred more than once a week with any activity limitation caused by asthma, and the relief medication was needed more than once a week or any night-time symptom occurred within the last past month, the case was not considered to be controlled based on the GINA guidelines. However, the NAEPP defines cases as uncontrolled when up to one night-time symptom occurs per month, daytime symptoms occur twice within a week, a short-acting β2 agonist for symptom control is required at least 2 days a week and/or there is at least one exacerbation within a year. In terms of asthma control, the requirement of the GINA-based assessment was higher than that of the NAEPP-based assessment for children under 5 years of age. TRACK was developed following the NAEPP asthma management guidelines, which explains the increased optimal cut-off point of 85 for the Chinese version of TRACK in our study. Overall, the above findings supported the pioneer report that indicated that TRACK scores below 80 can identify children with uncontrolled asthma or respiratory symptoms.
The main limitation of our study was that the caregivers had relatively high educational backgrounds. Although this study was a multicentre cohort study, it was mainly limited to Shanghai, Hangzhou and Nanjing. Most of the caregivers were from the above or nearby cities. As these are the most developed cities in China, the educational level is relatively higher than that in underdeveloped mid-west areas. The Chinese version of TRACK, which should be promoted in China in the future, needs to be further validated using different levels of regional participation in the country. Due to the limited number of cases and regional constraints, the optimal cut-off point for this test may not fully represent China as a whole.
In conclusion, these findings demonstrate the reliability and validity of the Chinese version of TRACK for assessing asthma control in children 5 years of age or younger. TRACK compensates for the insufficiency of other assessment tools for preschool-aged children in China. The promotion and application of TRACK in China could help caregivers and physicians evaluate the level of asthma control in children conveniently and effectively and further guide clinical treatment.
Acknowledgments
The authors would like to thank AstraZeneca Investments Limited (China), the China Pediatric Asthma Prevention and Management Program and Project Hope.
References
Footnotes
Contributors JGH, YY and LBZ contributed to the conception and design of the study, revising the draft critically for important intellectual content. JZ and SHL contributed to the data analysis and drafting of the submitted article. JGH and JZ contributed to the translation, data acquisition and interpretation of the outcomes. JGH and YY contributed to the crucial revision of the draft for important intellectual content, provided final confirmation of the revised version to be published and obtained permission to use the original questionnaire. DYZ, ZMC, HZ, JZ, LZ, SHY, MYT, YFW, WWZ, JX, LXZ, SYL and LZ contributed to the follow-up of patients and extracting and analysing the data. All authors contributed to the data analysis, drafting the manuscript and amending the paper and all take responsibility for all aspects of the work. All the data were accessible to all the authors to ensure the accuracy of the reported data.
Funding This study was funded by AstraZeneca China, the Shanghai Shen Kang Hospital Development Center’s Appropriate Technology Joint Development and Popularization Project 'The promotion of nebulization technology based on the standardized management of asthma in family and community for children' (no. SHDC12016216) and the Shanghai Shen Kang Hospital Development Center Projects for the Prevention and Control of Chronic Disease (no. SHDC12015306). It was also funded by the key projects of the Shanghai Science and Technology Department of Medicine (no. 16411955100), the Three-Year Action Plan to Promote Clinical Skills and Clinical Innovation Capability in Municipal Hospitals (no. 16CR4002A), the Discipline Construction of Nursing Plateau (no. hlgy16030kyg8), the Study on Schema Design for Enhancing the Compliance of ICS in Asthmatic Children Based on an Information Platform (no. jyh1519), the Important Weak Discipline of Shanghai Health and Family Planning Commission—Pediatrics (no. 2016ZB0104), the Shanghai Pudong New Area Municipal Planning Commission of Science and Research Fund (no. PW2017E-1), Projects for the construction of important and weak subjects in the health system of Pudong District (no. PWZbr2017-25) and the Key Project of China Hospital Development Institute of Shanghai Jiaotong University (CHDI-2017-A-06).
Competing interests None declared.
Patient consent for publication Parental/guardian consent obtained.
Ethics approval Ethical approval for the study was granted by the Shanghai General Hospital Research Ethics Committee (2018KY001) and Shanghai Children’s Medical Center Research Ethics Committee (SCMCIRB-K2016037).
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available on reasonable request.