Article Text
Abstract
Objectives Hyperuricemia and obesity both play a role in the development of hypertension. However, limited evidence is available for the combined effect of hyperuricemia and obesity on the prevalence of hypertension in the Chinese population. We aimed to assess the separate and combined effects of these two risk factors on the risk of hypertension.
Methods We conducted a cross-sectional study in an area of Dalian city, Liaoning Province, China, from September 2015 to November 2016; 8700 adult residents were invited to participate in this study. Hyperuricemia was defined as serum uric acid ≥ 416 μmol/L in men and ≥ 357 μmol/L in women according to the guidelines. Individuals were categorised into four groups: the control group (body mass index (BMI) §amp;lt; 25 without hyperuricemia, the reference group), the obesity group (BMI ≥ 25 without hyperuricemia), the hyperuricemia group (BMI §amp;lt; 25 with hyperuricemia) and the obese-hyperuricemia group (BMI ≥ 25 with hyperuricemia). A multivariable logistic model was used to investigate individual and combined effects of hyperuricemia and obesity on the risk of hypertension.
Results Of the 8331 individuals included, 44.3% were obese, 13.6% suffered from hyperuricemia, and 7.8% were both obese and hyperuricemic. The hypertension prevalence was the highest in the obese-hyperuricemia group (55.5% (95% CI 51.6% to 59.2%)), followed by that in the obesity (44.3% (42.6% to 46.1%)) and that in the hyperuricemia groups (33.5% (29.5% to 37.9%)). After adjusting for confounders, the obese-hyperuricemia group had a nearly threefold increased risk of hypertension compared with their healthy counterparts (OR 2.98 (2.48 to 3.57)). This pattern was also observed in the obesity group with a higher risk of hypertension (OR 2.18 (1.96 to 2.42)) compared with the control group, whereas the risk of hypertension was not elevated significantly in the hyperuricemia group (OR 1.14 (0.92 to 1.42)).
Conclusion Our study provided the first evidence that obese Chinese individuals with hyperuricemia had a significantly increased risk of hypertension compared with their healthy counterparts. This combined effect on the risk of hypertension is much stronger than the individual effect of either factor.
- hypertension
- hyperuricemia
- obesity
- community-dwelling subjects
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
The strengths of our study are the population-based design, the rigorous quality assurance programme and the large sample size.
The use of a population-based design minimises the possibility of sample selection bias.
The cross-sectional design does not explicitly imply a causal relation.
Introduction
Hypertension is now one of the most important issues for public health worldwide because of its increasing prevalence.1–3 Recent data reports indicate that 24.1% of adults were diagnosed with hypertension globally in 2015, and the estimated number of adults with elevated blood pressure increased from 594 million in 1975 to 1.13 billion in 20154; in China, among Chinese middle-aged adults (aged 32–75 years), the prevalence was up to 45% in 2017, and fewer than a third were being treated.5 Hypertension is a well-recognised major risk factor for stroke, cardiovascular disease, end-stage renal disease and overall mortality that affects all segments of the population,6 7 and its complications contribute to approximately one-third of the deaths due to cardiovascular diseases among the Chinese population.8 Therefore, appropriate treatment, along with efficacy strategies for the prevention and identification of subjects at high risk, should be implemented to modify these trends.
Effective prevention and control of hypertension relies on great progress in our understanding of the risk factors for hypertension. Despite the fact that the causal effect of serum uric acid (SUA) on hypertension remains controversial, numerous prospective studies have recently demonstrated that an elevated SUA (hyperuricemia) may be an independent risk factor for developing prehypertension, primary hypertension and resistant hypertension in various populations.9–11 This evidence is further supported and justified by several animal experiments reporting that an increasing uric acid level in rats causes hypertension.12 13 Moreover, according to pilot clinical studies, SUA-lowering therapy may have benefit on reducing blood pressure (BP) in hypertensive and prehypertensive children and adults.14 The potential mechanisms underlying this hyperuricemia–hypertension link may be diverse, such as nitric oxide synthase–related endothelial dysfunction, renin–angiotensin system (RAS) activation and the stimulated proliferation of vascular smooth muscle cells.12 15 16
Although growing evidence has supported an important role of elevated SUA in the development of hypertension, the association between SUA and hypertension is confounded by numerous factors; hence, the significance of their association remains controversial. For instance, elevated SUA or hyperuricemia is also observed in obese subjects, which in turn affects the development of hypertension.17 18 Obesity, another increasing prevalent condition, is recognised as the main cause of various diseases,19–23 and it is also an established risk factor for the development of hyperuricemia. Obesity or excess body fat may be related to SUA overproduction and poor SUA excretion, which leads to impaired uric acid metabolism or even hyperuricemia,24 whereas weight loss may be effective in preventing the decreasing SUA levels, especially in postmenopausal women and in men.25 Thus, it is still difficult to confirm the involvement of elevated SUA levels/hyperuricemia in the pathogenesis of hypertension because the association between hyperuricemia and hypertension risk may be different according to body mass index (BMI) levels; therefore, it is important for clinical studies that assess this association to take obesity status into account.
To date, as far as we know, no large-scale studies have investigated the association between hyperuricemia and hypertension by considering obesity status in the Chinese population. Therefore, in the present study, we aimed to fill this gap in knowledge and examine the separate and combined effects of hyperuricemia and obesity on the risk of hypertension among different sex and age groups, especially assessing to what extent obesity modifies these associations in a population-level study of Chinese adults.
Methods
Study population
This study used data from a community-based survey conducted in Dalian city, Liaoning Province, China, from September 2015 to November 2016, and the study aimed to investigate the prevalence of and risk factors for hypertension in Liaoning Province, China. The study used a stratified random cluster sampling method. In the first stage of sampling, three districts were selected randomly in Dalian; in the second stage of sampling, four communities were subsequently selected randomly from each district; in the third stage of sampling, all eligible permanent residents aged ≥18 years in each chosen communities were selected and invited to participate in the study. Participants were included if (1) they lived in the selected communities for at least 5 years, (2) they were aged ≥18 years and (3) willing to participate in this survey. Participants were excluded if they were (1) unable to answer the questionnaire, (2) unable to complete blood sampling or anthropometric or blood pressure measurements, and (3) known to have severe psychological disorders, Alzheimer’s disease, dementia or other infectious disease. The sample size for the present study was calculated based on a prevalence (p) of hypertension of 25% among adults aged ≥18 years in Liaoning Province, the design effect (deff) of 1.5, a u-value of 1.96, a relative error (r) of 5% and a non-response rate of 20%, using the formula n=deff×u2(p(1 p)/(r×p)2)×(1+20%). We estimated a required sample size of approximately 8700 participants. Of those 8700 participants randomly invited to the study, 8331 participants agreed to and completed the present study; the overall response rate was 95.8% (8331/8700).
Data collection and measurement
The survey involved a questionnaire interview, physical examination and biochemical measurements. The standardised questionnaire was completed by the participants in a face-to-face interview with well-trained personnel. Standardised questionnaire included information on sociodemographic characteristics (age, gender, marital status and education level), behavioural factors (smoking status, alcohol intake, physical activity), and their medications and self-reported family histories.
The physical examination evaluated anthropometric measurement and BP. Body weight and height were measured while participants were barefoot and dressed in light clothing and were measured to the nearest 0.1 kg and 0.1 cm, respectively. Waist circumference was measured midway between the lowest rib and the iliac crest with flexible anthropometric tape on the horizontal plane with the participant in the standing position. Hip circumference was measured over thin clothing at the point of the maximum circumference of the buttocks. Both circumferences were measured to the nearest 0.1 cm. BMI was calculated by dividing weight (in kilograms) by the square of height (in metres). Systolic and diastolic blood pressures (SBP and DBP) were measured on the right arm using mercury sphygmomanometers. Measurements were collected in triplicate after a 10 min seated rest, and the mean of the three measurements was used in our analyses.
Following an overnight fast, fasting blood samples were collected in the morning. Plasma and serum samples were then frozen and stored at −86°C for later laboratory analysis. All blood samples were analysed at a central, certified laboratory in Dalian with strict quality control. Serum levels of fasting plasma glucose, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) concentrations, triglycerides (TG), SUA and other routine blood biochemical indices were measured by a biochemical autoanalyser.
Definition of outcome variable
According to the criteria recommended by the US Joint National Committee and Chinese guidelines,26 27 hypertension was defined as an SBP ≥140 mm Hg, a DBP ≥90 mm Hg and/or the self-reported use of antihypertensive medication.
Definition of exposure variables
Obesity status was defined by using a BMI cut-off point of 25 kg/m2, according to the definition advocated by Western Pacific Regional Office of WHO for obesity in Asian adults.28 29 In our study, hyperuricemia was a defined as an SUA ≥ 416/357 μmol/L in men and women, according to the guidelines.30 31
Definition of sociodemographic covariates
The sociodemographic covariates consisted of sex (male and female), age, marital status (single, married, windowed) and educational level (primary school or below, middle school and high school or above). Behavioural covariates included smoking status (yes (current or former), no), alcohol intake (yes (current or former), no) and physical activity (>60 min/day vs 30–60 min/day vs <30 min/day vs none; >60 min/day and 30–60 min/day were considered active physical activity, and <30 min/day and none were considered inactive physical activity). Family history and medication variables included a personal history of diabetes (yes, no), heart failure (yes, no) and coronary heart disease (CHD) (yes, no); family medical history (none, hypertension, CHD, diabetes or others); hypertension awareness (yes, no); BP control (yes, no) and the use of hypertension medication (yes, no); and the type of hypertension treatment (calcium channel blocker, ACE inhibitor, angiotensin receptor blocker or others).
Statistical analyses
The characteristics of the study population were presented as the mean and SD for continuous variables, or as numbers and percentages for categorical variables. Individuals were categorised into four groups: the control group (BMI §amp;lt; 25 without hyperuricemia, the reference group), the obesity group (BMI ≥ 25 without hyperuricemia), the hyperuricemia group (BMI §amp;lt; 25 with hyperuricemia) and the obese-hyperuricemia group (BMI ≥ 25 with hyperuricemia). The assumption of normality was examined with the Shapiro-Wilk test, and the assumption of homoscedasticity was examined by Levene’s test. Comparison of the four groups was determined using one-way analysis of variance (ANOVA) tests or Kruskal-Wallis test (if the assumptions of ANOVA are not met: normality and homoscedasticity studied by Levene’s test) for continuous variables, or χ2 test for categorical variables. The association among obesity, hyperuricemia and hypertension was examined by multivariate logistic regression models, with ORs and 95% CIs calculated. The ORs and their 95% CIs for the presence of hyperuricemia were first adjusted for age and sex (model 1), and then further adjusted for smoking status, alcohol intake, education level, physical activity, TC, TG, urea and creatinine (model 2). The control group was used as the reference in these analyses. To make our results solid, a sensitivity analysis was performed by using alternative definition of hyperuricemia, namely, a SUA level ≥420 μmol/L in men and ≥360 μmol/L in women, and the main findings are presented in online supplement tables. All statistical analyses were conducted with R V.3.2.2 software (R Foundation for Statistical Computing, Vienna, Austria),32 and a p value <0.05 was considered statistically significant.
Patient and public involvement
Patients were not involved in this study.
Results
Characteristics of the study sample
The characteristics of the study sample are presented in table 1. A total of 8331 individuals were included, of whom 3694 (44.3%) were obese, 1131 (13.6%) suffered from hyperuricemia, and 651 (7.8%) had both obesity and hyperuricemia. Individuals were divided into four groups: 4157 non-obese without hyperuricemia, 3043 obese without hyperuricemia, 480 non-obese with hyperuricemia and 651 obese with hyperuricemia (table 1). The individuals with hyperuricemia were significantly older than their counterparts without hyperuricemia, but there were no significant differences in age between the control group and the obesity group. Moreover, the four groups had very similar heights. The obese groups had higher anthropometric parameters and an elevated BP compared with the non-obese groups, with the highest anthropometric values found in the obese-hyperuricemia group. Compared with individuals without hyperuricemia, individuals with hypeuricemia were more likely to exhibit a less favourable risk profile, such as elevated TC, HDL-C, urea and creatinine levels, regardless of BMI level. However, individuals within both non-obese groups (the control and hyperuricemia groups) were likely to be men, smokers and drinkers, compared with their obese counterparts.
Study sample characteristics of subjects according to obesity status (defined by body mass index) and hyperuricemia
Prevalence of hypertension
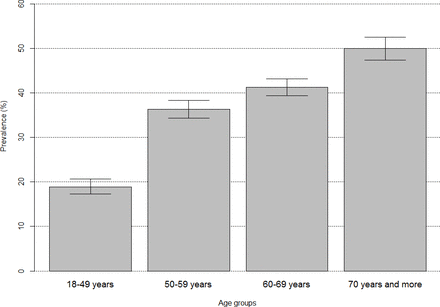
The overall crude prevalence of hypertension was 36.0% (95% CI 35.0% to 37.1%) in the study sample, with sex-specific prevalence of 36.4% (34.5% to 38.3%) and 35.9% (34.6% to 37.1%) in men and women, respectively. The prevalence of hypertension among Chinese adults with obesity was significantly higher (46.3%, 95% CI 44.7% to 47.9%) than that of their counterparts with normal weight (27.8%, 95% CI 26.6% to 29.1%), and a significantly higher prevalence was found among adults with hyperuricemia (46.2%, 95% CI 43.3% to 49.1%) compared with their counterparts without hyperuricemia (34.4%, 95% CI 33.3% to 35.3%). In addition, the hypertension prevalence increased as age progressed, ranging from 18.9% (95% CI 17.3% to 20.7%) for the group aged 18–49 years to 50% (47.4%–52.5%) for the group aged 70 years and above (figure 1).
Prevalence of hypertension by age group.
Prevalence of hypertension with the combination of hyperuricemia and obesity
Figure 2 shows the impact of obesity, hyperuricemia and none or both of these risk factors with the prevalence of hypertension. The overall prevalence of hypertension was 55.5% (95% CI 51.6% to 59.2%) in the obese-hyperuricemia group, which was significantly higher than that in the obesity (44.3%, 95% CI 42.6% to 46.1%) and hyperuricemia groups (33.5%, 95% CI 29.5% to 37.9%) (figure 2). The crude prevalence of hypertension among both the obesity group (44.3%, 95% CI 42.6% to 46.1%) and the hyperuricemia group (33.5%, 95% CI 29.5% to 37.9%) was significantly higher than that in the groups without obesity or hyperuricemia (27.2%, 95% CI 25.9 to 28.6) (table 2).
Prevalence of hypertension by combining of obesity and hyperuricemia status. The obesity status was defined by body mass index with cut-off point of 25 kg/m2. (A) Overall prevalence in four groups. (B) Sex-specific prevalence in four groups.
Prevalence of hypertension stratified by sex and age groups
In both men and women, the highest prevalence of hypertension was consistently found among obese individuals with hyperuricemia, with a prevalence of 59.3% (95% CI 51.8% to 66.4%) and 54.1% (95% CI 49.6% to 58.5%), respectively, followed by the prevalence in the obesity group (47.1%, 95% CI 43.6% to 50.7% for men and 43.4, 95% CI 41.4% to 45.4% for women). Moreover, these prevalence rates were significantly higher than those in the hyperuricemia and control groups. Within different obesity and hyperuricemia groups, the male individuals were more likely to have hypertension than the female individuals; however, the difference was not significant (figure 2).
The prevalence of hypertension increased with age regardless of obesity and hyperuricemia groups. The highest prevalence of hypertension was consistently found in the obesity-hyperuricemia group, ranging from 34.1% in individuals aged 18–49 years to 72.1% among individuals aged ≥ 70 years, respectively. The obesity group revealed a higher prevalence of hypertension for all different age groups than did the hyperuricemia group, with hypertension affecting nearly one-fourth of the obese individuals without hyperuricemia aged 18–49 years and more than half of those aged >70 years suffering from hyperuricemia. In addition, among individuals without obesity and hyperuricemia, the prevalence of hypertension still reached 25.1% for participants aged 50–59 years and 42.5% for participants who were aged at least 70 years. A sensitivity analysis, using a SUA threshold of ≥ 420 μmol/L in men and ≥ 360 μmol/L in women to define hyperuricemia, showed highly consistent results on prevalence of hypertension (online supplementary table 1).
Supplemental material
Model 1: associations among hypertension, obesity and hyperuricemia
Figure 3 shows the association of obesity, hyperuricemia and none or both of these risk factors with the prevalence of hypertension. The age-adjusted and sex-adjusted ORs (95% CIs) for hypertension were 3.43 (2.87 to 4.08) for the obese-hyperuricemia group, 2.28 (2.06 to 2.53) for the obesity group and 1.26 (95% CI 1.03 to 1.56) for the hyperuricemia group when compared with the control group (model 1), which demonstrates a strong association among hypertension, obesity and hyperuricemia, particularly when considering the combined effects of obesity and hyperuricemia (figure 3). When taking into account the sex-specific effects and age groups, a similar pattern was also found (table 3).
Adjusted OR and 95% CI for hypertension risk associated obesity, hyperuricemia and none or both of these two risk factors. Horizontal bars are 95% CIs. The adjusted OR was obtained from model 1 and model 2. Model 1: adjusted for age and sex. Model 2: adjusted for model 1+smoking status, alcohol drinking status, health education, physical activity, total cholesterol, triglycerides, urea and creatinine.
Adjusted ORs and 95% CIs of prevalence for the hypertension with combination of obesity and hyperuricemia
Model 2: associations among hypertension, obesity and hyperuricemia
After adjusting for age, sex, lifestyle and other confounders (table 3, model 2), the association between hypertension and obesity and/or hyperuricemia status was attenuated but still highly significant, except for hyperuricemia group (OR 1.14, 95% CI 0.92 to 1.42). Participants in the obese-hyperuricemia group were nearly threefold more likely to have hypertension (OR 2.98, 95% CI 2.48 to 3.57), and the obese individuals without hyperuricemia had an approximately twofold increased risk (OR 2.18, 95% CI 1.96 to 2.42) compared with their healthy peers. Generally, a similar pattern was noted when the analysis was stratified in both sexes, but with a consistently higher magnitude of OR values in men than women, irrespective of obesity and hyperuricemia status. With respect to different age groups, individuals with only obesity demonstrated a significant twofold increased risk of hypertension compared with their healthy peers regardless of age group, and the risk of having hypertension increased to threefold among obese individuals who also had hyperuricemia, with ORs ranging from 2.48 to 3.12. Furthermore, the magnitude of the ORs from the obesity group decreased with age, whereas the obese-hyperuricemia group demonstrated a U-shaped relationship with hypertension. In addition, the effect of hyperuricemia on hypertension was only significantly seen among individuals aged 50–59 years (OR 1.93, 95% CI 1.27 to 2.95).
Furthermore, when using another hyperuricemia criteria (the sensitivity analysis), a highly similar pattern was found: the strength of association between separate and combined effects of obesity and hyperuricemia and risk of hypertension was almost identical (online supplementary table 2).
Discussion
To the best of our knowledge, this is the first large-scale study of the combined effect of hyperuricemia and obesity on the prevalence of hypertension among Chinese adults. We show that the combination of hyperuricemia and obesity significantly increases the prevalence of hypertension among a Chinese population to a greater extent than either of the two factors alone. A significant positive association between hyperuricemia and hypertension was only observed in obese Chinese individuals, but not in normal-weight individuals, which suggests a modifying effect of obesity on the association. Furthermore, the individual and combined effects of hyperuricemia and obesity on the prevalence of hypertension varied among different sex and age groups.
Several recent prospective studies have focused on the risk of hypertension incidence associated with an elevated SUA level (hyperuricemia). In an early cohort study of 4489 Japanese subjects free of hypertension after a follow-up of 3 years, Nagahama et al found that subjects with hyperuricemia at baseline had an increased risk of hypertension compared with their counterparts without hyperuricemia, with an adjusted OR of 1.48 and 1.90 for men and women, respectively.33 Subjects with hyperuricemia also showed a significantly greater increase in SBP during the 3 year follow-up, with the increase more pronounced in women. Another cohort study that included 608 non-hypertensive Chinese adults found a nearly doubled risk of hypertension among individuals in the highest SUA quartile group compared with those in the lowest quartile group; the increased risk was most pronounced for those with pre-hypertension at the start of the study.34 In a community-based study that included 580 Italians over the age of 65, Mazza et al demonstrated that an SUA value of ≥6.8 mg/dL tripled the risk of resistant hypertension in elderly women, but not in men. This finding emphasises the value of SUA assessments as a way to define the risk patterns associated with resistant hypertension.35 Similar findings were recorded in another large prospective study performed among Chinese adults.36 The cumulative incidence of hypertension was consistently higher among individuals with hyperuricemia than among those with normal SUA levels; subjects in the quartile with the highest SUA levels had a risk of hypertension that was approximately three times higher than those in the lowest quartile after controlling for age, sex and biomarkers. Of note, a significant dose–response relationship was observed between the SUA quartile and the incidence of hypertension, with augmentation of the risk of hypertension for the upper SUA quartile.36 Moreover, two recent meta-analyses that included 18 and 25 prospective cohort studies confirmed that hyperuricemia was associated with an increase in the risk of developing hypertension by a factor of 1.5 and supported the existence of a dose–response relationship; an increase in risk of 15% was noted for each increase in SUA of 1 mg/dL, and the risk increased by 19% for every 1 SD increase.37 38
Hyperuricemia and obesity are well-known risk factors for hypertension, and there is a positive association between obesity and hyperuricemia. Previous studies have shown that an increase in visceral fat accumulation provides an overflow of free fatty acids to the liver and visceral adipose tissues that induce excessive SUA production.24 39 In addition, the pentose phosphate pathway provides an excessive in flow of free fatty acids that may be linked to de novo purine synthesis, which, in turn, accelerates UA production.40 41 Another plausible mechanism for the link with obesity is a reduction in the extrarenal excretion of UA related to visceral fat accumulation. Numerous investigators have suggested that visceral adipose tissue is pathologically active and impairs the regulation of adipocytokine release. Adipocyte dysregulation is believed to alter the transport of uric acid in the renal tubules, thereby reducing urinary excretion and urinary sodium excretion, which leads to hyperuricemia.42–45 Moreover, using a bidirectional Mendelian randomisation approach, Lyngdoh et al found that adiposity markers explained by genetic variants were positively and significantly associated with SUA, whereas SUA explained by a proxy of a gene instrument the SLC2A9 was not associated with fat mass.46 The evidence for causality is strong because of the Mendelian analysis, and suggests that elevated SUA is a consequence, rather than a cause, of adiposity. Therefore, obesity may be considered a mediator of the causal pathway between hyperuricemia/elevated SUA and hypertension.
Until recently, few studies have focused on the association between SUA and the risk of hypertension for different BMI levels or obesity statuses. Based on the data from NHANES 1999–2012, which included 31 473 adult participants, Han et al found that more than half of the participants with both hyperuricemia and obesity were classified as hypertensive, which was significantly higher than among those with hyperuricemia (41.7%) or obesity (30.6%) alone. More importantly, the obese subjects with hyperuricemia had a fourfold increase in the risk of hypertension compared with their healthy peers, and the magnitude of the combined risk factor was much higher than any of the single risk factors.17 Similar findings were reported for a prospective cohort study of Norwegian subjects who participated in population-based surveys.18 A 7-year follow-up survey of this cohort showed that baseline UA was an independent predictor of hypertension in the overweight group, with an increase in the OR of 1.44 per 59 μmol/L UA. The association between the BMI-cut off and UA for the prediction of new cases of elevated blood pressure was also significant (p=0.04). However, the significance was lost when subjects had normal weight, suggesting that obesity modified the association between baseline UA and the incidence of elevated BP over the course of the longitudinal study. Our results are consistent with these previous findings and show that the risk of hypertension for obese subjects with hyperuricemia increases by a factor of two, and that this result is statistically significant. A non-significant association was found among the normal-weight subjects with hyperuricemia. In contrast, a small study of 69 young women from the United Arab Emirates (UAE) reported that the correlation between UA and BP was not confined to the obese subjects.47 The apparent contradiction between this study and the others might be attributable to the small sample size of the UAE study, and different characteristics among the study populations.
The mechanism by which BMI modifies the association between SUA and hypertension is not fully understood. However, several studies have suggested a role for adipocytes. It has been proposed that elevated SUA decreases regulation of the adiponectin,48 which is an anti-inflammatory factor known to be negatively related to BMI and body fat.49–51 Low levels of adiponectin are associated with an increased risk of hypertension development,52 so adiponectin might form part of the linkage between SUA and elevated blood pressure. This would help to explain the mechanism by which BMI or obesity modifies the association between SUA and hypertension. Our results support an intermediary role for obesity in the causal pathway between hyperuricemia and hypertension. When all adult subjects were considered, hyperuricemia accounted for 6.3% (33.5%–27.2%) of the difference in hypertension between the control group and the hyperuricemia group, while obesity accounted for 22% (55.5%–33.5%) of the difference in hypertension between the hyperuricemia group and the group with combined hyperuricemia and obesity.
It is known that hyperuricemia is an important risk factor for hypertension and that renal involvement provides the causal pathway that leads to the development of hypertension. However, the use of urate-lowering therapies to treat hyperuricemia and thereby prevent hypertension is still at an early stage. The results of experimental animal studies showed that urate-lowering therapies could reduce SUA levels and decrease BP in rats with pre-existing hypertension by activating RAS and decreasing endothelial nitric oxide synthase.12 The first two rigorously designed randomised trials with a blinded placebo-controlled study design were performed by Feig, Soletsky and colleagues. The experiments involved 30 hypertensive adolescents and 60 obese non-hypertensive adolescents. Consistent and significant reductions in the mean 24 hours SBP of 6.3 mm Hg were observed, and SBP reductions of 8.9–9.2 mm Hg were achieved after 4–8 weeks of urate-lowering therapy with either allopurinol or probenecid.9 10 A larger BP reduction was observed among those without hypertension at the baseline and milder hyperuricemia (SUA levels ≥ 5.0 mg/dL). The use of uric acid reduction for the relief of hypertension among adolescents is promising, and its efficacy has been supported by small clinical trials. In a small prospective study of 48 hyperuricemic patients conducted by Kanbay et al , an allopurinol-based treatment for hyperuricemia was associated with a significant decrease in both SBP and DBP levels at the 3 month follow-up; this finding supports the idea of an antihypertensive effect for urate-lowering therapy.53 The evidence therefore suggests that, at least for adolescents, subjects with more moderate uric acid and normal BP levels are the most susceptible to BP lowering in response to uric acid reduction. However, a small number of studies have provided results that are inconsistent with the evidence from these animal experiments and clinical trials. In a recent double-blind placebo-controlled trial that included 149 overweight/obese adults with high SUA, McMullan et al demonstrated that uric acid-lowering therapy with either allopurinol (43%) or probenecid (30 %) had no effect on kidney-specific or systemic RAS activity after 8 weeks, and no effect on mean SBP.54 Thus, further evidence is still required to guide therapeutic decisions for adults with hyperuricemia. Urate-lowering drugs can have serious side effects; therefore, the identification of suitable target populations for further intervention is important for clinical trial design. Our results showed that hyperuricemia was not significantly associated with the prevalence of hypertension among normal-weight Chinese subjects, and that a significant effect was only found among subjects aged 50–59 years.
Strengths and limitations
There were several limitations to the present study. First, the cross-sectional design meant that it was not possible to determine the causality among hyperuricemia, obesity and hypertension risk; further cohort studies are warranted to clarify our findings. Second, our study population included only Chinese subjects, so extrapolation of the results to other ethnic groups should be undertaken with caution. Nevertheless, the population-based design of our study, the rigorous quality assurance programme and the large sample size mean that this study produced statistically valid results and that the associations between the parameters of interest are likely to be robust. In addition, the use of a population-based design minimises the possibility of sample selection bias. Finally, our models were adjusted for covariates including age, sex, alcohol consumption, smoking and other serum measurements to minimise confounding effects.
Conclusion
The combined effect of hyperuricemia and obesity on hypertension risk is stronger than the individual effects of the two factors for a population-based Chinese cohort. No significant association was found among normal-weight hyperuricemic subjects, suggesting a modifying effect of obesity on the relationship between hyperuricemia and hypertension risk. These findings provide insights that will improve the design of further cohort studies of the causal relationship between hyperuricemia and hypertension risk as a function of BMI level and will help to identify appropriate target populations for specific treatment strategies.
Acknowledgments
The authors would like to thank all the participants for their contribution and participation, and to thank the experts from AJE for manuscript’s English language editing.
References
Footnotes
Contributors ST: conceived the study, designed the study, performed statistical analysis and wrote the paper. YL: conceived the study, designed the study, collected the data and wrote the paper. YX: collected the data. AF: collected the data and performed statistical analysis. All authors reviewed and approved the final manuscript for submission.
Funding This research was funded by National Natural Science Foundation of China (81803329) and China Postdoctoral Science Foundation (2018M631780).
Competing interests None declared.
Patient consent for publication Obtained.
Ethics approval The study was approved by the Ethics Committee of the Affiliated Zhongshan hospital of Dalian University (Dalian, China), and in accordance to the Declaration of Helsinki. All procedures were performed in accordance with the ethical standards.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available on reasonable request.