Article Text
Abstract
Objective Studies on the association between clinical vertebral fractures (CVFs) and the subsequent risk of cardiopulmonary diseases, including aortic dissection (AD), congestive heart failure (CHF), pneumonia and acute respiratory distress syndrome (ARDS) are scarce. Therefore, we used the National Health Insurance Research Database to investigate whether patients with CVF have a heightened risk of subsequent AD, CHF, pneumonia and ARDS.
Design The National Health Insurance Research Database was used to investigate whether patients with CVFs have an increased risk of subsequent AD, CHF, pneumonia and ARDS.
Participants This cohort study comprised patients aged ≥18 years with a diagnosis of CVF and were hospitalised at any point during 2000–2010 (n=1 08 935). Each CVF patient was frequency-matched to a no-CVF hospitalised patients based on age, sex, index year and comorbidities (n=1 08 935). The Cox proportional hazard regressions model was used to estimate the adjusted effect of CVF on AD, CHF, pneumonia and ARDS risk.
Results The overall incidence of AD, CHF, pneumonia and ARDS was higher in the CVF group than in the no-CVF group (4.85 vs 3.99, 119.1 vs 89.6, 283.3 vs 183.5 and 9.18 vs 4.18/10 000 person-years, respectively). After adjustment for age, sex, comorbidities and Charlson comorbidity index score, patients with CVF had a 1.23-fold higher risk of AD (95% CI=1.03–1.45), 1.35-fold higher risk of CHF (95% CI=1.30–1.40), 1.57-fold higher risk of pneumonia (95% CI=1.54–1.61) and 2.21-fold higher risk of ARDS (95% CI=1.91–2.57) than did those without CVF. Patients with cervical CVF and SCI were more likely to develop pneumonia and ARDS.
Conclusions Our study demonstrates that CVFs are associated with an increased risk of subsequent cardiopulmonary diseases. Future investigations are encouraged to delineate the mechanisms underlying this association.
- clinical vertebral fracture
- aortic dissection
- congestive heart failure
- pneumonia
- acute respiratory distress syndrome
- national health insurance research database
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- clinical vertebral fracture
- aortic dissection
- congestive heart failure
- pneumonia
- acute respiratory distress syndrome
- national health insurance research database
Strengths and limitations of this study
This is the first population-based, longitudinal cohort study to focus on the correlation between CVF and the subsequent risks of specific cardiopulmonary diseases.
By sampling from a large nationwide database, which covers nearly 100% of all residents in Taiwan, stable outcomes could be achieved with such adequate, representative samples.
All disease definitions and sample selection in our study were based on the ICD-9-CM coding. Therefore, miscoding or misclassification might exist, although it is considered rare.
In our study, sampled participants were retrieved from NHIRD from January 1, 2000, to December 31, 2010. Aging property of the data might not truly reflect the current medical conditions.
Because of geographic and epidemiologic discrepancies, our results might not be applicable to other countries or regions.
Introduction
Clinical Vertebral fractures (CVFs) constitute a major healthcare burden worldwide because of its high incidence and strong influence on individuals’ quality of life, medical resource consumption and direct or potential unfavourable impacts on socioeconomic development.1–3 Approximately 1.4 million new cases of CVF are diagnosed globally every year,4 and among these, osteoporosis, trauma and malignancy are the major etiologies.5–9 Acute aortic dissection (AD) remains the major life-threatening vascular emergency, with a steadily increasing incidence because of population ageing and the explosive growth of radiologic technology.10–12 Without early recognition and timely treatment, the prognosis of AD would be extremely poor, and half the patients would die within 48 h10. Congestive heart failure (CHF) is the major cause of hospitalisation in old age, with more than 650 000 new cases confirmed annually in the United States, and more than 1 million people were hospitalised for decompensated CHF, resulting in costs exceeding 39 billion13–15. Pneumonia is one of the most common infectious diseases in elderly adults and is also the leading cause of death in Americans older than 65 years16.17 Acute respiratory distress syndrome (ARDS) is a complex syndrome characterised by diffuse hydrostatic pulmonary oedema, alveoli damage and persistent hypoxemia, which are mainly triggered by infection, inflammation, trauma, or other etiologies. The in-hospital mortality rate for this condition could reach 40% even when managed with the standardised lung protective ventilator strategy.18 19
Studies have demonstrated that elderly patients with a history of osteoporotic vertebral fracture have an increased risk of cardiovascular events, including stroke (ischaemic or haemorrhagic) and coronary heart disease.20–23 Recently, Kim et al 24 reported an association between isolated CVF and future development of pneumonia in women with low bone density. In addition, chronic, worsened and longstanding backache accompanied with CVF might result in a long-term increase of sympathetic tone, fatigue, stress reaction, low physical activity, depressive tendency, diminished pulmonary function and, consequently a poor quality of life, which might be correlated with cardiopulmonary disease risk.3 5 7 8 25 Therefore, we hypothesised that an association exists between CVF and the risk of cardiopulmonary diseases, including AD, CHF, pneumonia and ARDS. Accordingly, we conducted a nationwide, population-based data analysis to verify this hypothesis and tried to provide essential evidence-based information for clinical practice.
Methods
Data source
This retrospective cohort study used datasets from Taiwan’s National Health Insurance Research Database (NHIRD). Taiwan launched a single-payer National Health Insurance (NHI) programme in March 1995, and 99% of the 23.74 million residents were enrolled.26 The details of the NHIRD and NHI programme are well presented in previous studies.27–33 The NHIRD records diseases according to International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Validation of the NHIRD with cardiovascular diseases were investigated and appeared to be a valid resource for population research.34–37 This study was approved by the Institutional Review Board of China Medical University Hospital (CMUH-104-REC2-115-CR4).
Sampled participants
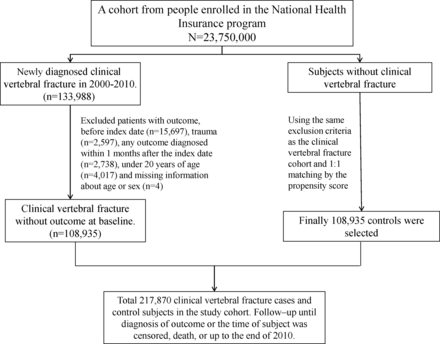
Patients aged ≥18 years with newly diagnosed CVF (ICD-9-CM codes, 805 and 806) from January 1, 2000, to December 31, 2010, were identified as the CVF cohort. Study subjects with the diagnosis of vertebral fracture from 1996 to 1999 were excluded at the baseline. The location of CVF was defined in two ways as follows: (1) cervical spine (ICD-9-CM codes, 805.0–805.18 and 806.0–806.19), thoracic spine (ICD-9-CM codes, 805.2, 805.3 and 806.2–806.39), lumbar spine (ICD-9-CM codes, 805.4, 805.5, 806.4 and 806.5) and sacrum plus coccyx (ICD-9-CM codes, 805.6, 805.7 and 806.6–806.79) and (2) without spinal cord injury (SCI) (ICD-9-CM codes, 805–805.9) and with SCI (ICD-9-CM codes, 806–806.9). The date of first-time CVF diagnosis at admission was defined as the index date. Participants with prior AD (ICD-9-CM codes, 441.0, 441.00, 441.01, 441.02 and 441.03), CHF (ICD-9-CM code, 428), pneumonia (ICD-9-CM codes, 480–488) and ARDS (ICD-9-CM codes, 518.82 and 518.5) before 1999 and before the index date (n=15 697); with the diagnosis of trauma (ICD-9-CM codes, 800–959 except 805–806) during the same period (n=2597); with any outcome event (AD, CHF, pneumonia and ARDS) diagnosed within 1 month after the index date (n=2738); those under 18 years of age (n=4017); and those with missing information about age or sex (n=4) in both the CVF and no-CVF cohorts; were excluded. For each CVF patient, a no-CVF participant was frequency-matched by the index year of CVF diagnosis, age (every 5 year span), sex and comorbidities of diabetes (ICD-9-CM code, 250), hypertension (ICD-9-CM codes, 401–405), hyperlipidemia (ICD-9-CM code, 272), atrial fibrillation (ICD-9-CM code, 427.31), chronic kidney disease (CKD; ICD-9-CM codes, 580–589) and chronic obstructive pulmonary disease (COPD; ICD-9-CM codes, 491, 492 and 496) (figure 1). Coexisting comorbidities were identified before the index date, with at least one time of principal or secondary diagnoses documented in hospitalizations during the period 2000 to 2010. We have also added Charlson comorbidity index (CCI) score as a confounding factor. Summary of ICD-9-CM codes applied for disease definition are presented in online supplementary table 1.
Supplemental material
Derivation of our study cohort.
Outcome
The main outcome was hospitalisation with a new diagnosis of AD, CHF, pneumonia, or ARDS during the follow-up period. Both the CVF and no-CVF cohorts were followed up until the diseases appeared or they were censored because of loss to follow-up, death, or the end of December 31, 2010, whichever occurred first.
Statistical analysis
A chi-square test and Student’s t-test were used to evaluate the differences in the distribution of categorical and continuous variables, respectively, between the CVF and no-CVF cohorts. The overall, sex-specific, age-specific and comorbidity-specific incidence densities of AD, CHF, pneumonia and ARDS were estimated for each cohort. To address the concern of constant proportionality, we examined the proportional hazard model assumption using a test of scaled Schoenfeld residuals. The results showed that there was no significant relationship between Schoenfeld residuals for CVF and follow-up time (p-value=0.06) in the model evaluating the AD risk and Schoenfeld residuals for CVF and follow-up time (p-value=0.18) in the model evaluating the ARDS risk. In the model evaluating the CHF and pneumonia risk throughout overall follow-up period, the results of the test revealed a significant relationship between Schoenfeld residuals for CVF and follow-up time, suggesting the proportionality assumption was violated. The relative risks of AD, CHF, pneumonia and ARDS in the CVF cohort compared with the no-CVF cohort were analysed using univariable and multivariable Cox proportional hazard regression models and presented as HRs and 95% CIs. The multivariable models were simultaneously adjusted for age, sex and comorbidities of hypertension, diabetes, hyperlipidemia, atrial fibrillation, CKD and COPD. We further tested the interaction between gender and VCF; between age and VCF; and between comorbidity and VCF by including a cross-product term in the model. Further analysis was performed to assess whether the association of CVF with AD, CHF, pneumonia and ARDS varied according to the levels of CVF. All statistical analyses were performed using SAS 9.4 software (SAS Institute, Cary, North Carolina, USA), and we set the significance level at less than 0.05 for two-sided testing of P values.
Patient and public involvement
There was no patient or public involvement in this study.
Results
Demographics and comorbidity
In this study, 108,935 CVF patients and 108 935 matched no-CVF participants with similar distributions of age, sex and comorbidities were assessed (table 1). In the CVF cohort,≥44.3% of patients were aged ≥65 years, and 55.3% of the patients were women (table 1). The mean age of the patients was 58.8±18.8 years in the CVF cohort and 58.3±18.8 years in the no-CVF cohort. Both cohorts had a medical history of hypertension (26.0%), diabetes (15.2%), COPD (5.3%), hyperlipidemia (5.2%), atrial fibrillation (1.2%) and CKD (3.5%). Patients of CVF cohort were more prevalent with CCI than no-CVF cohort.
Comparison of demographics and comorbidity between clinical vertebral fracture patients and controls
Primary outcomes
Overall, the incidence of AD was 1.22 -fold higher in the CVF cohort than in the no-CVF cohort (4.85 vs 3.99 per 10 000 person-years), with an adjusted HR (aHR) of 1.23 (95% CI=1.03–1.45) (table 2). The aHR of AD among women was significantly higher in the CVF cohort than in the no-CVF cohort (aHR=1.40, 95% CI=1.09–1.79). The age-specific relative hazard of AD in the CVF cohort was higher than that in the no-CVF cohort for age ≤49 group. The relative hazard of AD was higher in the CVF cohort than in the no-CVF cohort for patients without comorbidities (aHR=1.38, 95% CI=1.04–1.83). In all stratifications, the risk of CHF, pneumonia and ARDS remained higher in the CVF cohort than in the no-CVF cohort.
Incidence and adjusted HR of outcome by sex, age and comorbidity for clinical vertebral fracture patients compared with controls
Subtypes analysis
Compared with patients without CVF, the risk of AD was 1.33-fold (95% CI=1.11–1.60) higher in CVF-lumbar patients and was 1.25-fold (95% CI=1.05–1.48) higher in CVF patients without SCI (table 3). The risk of CHF and pneumonia remained higher in patients with various levels of CVF than in patients without CVF. table 3 also shows that patients with various levels of CVF, except for those with sacrum or coccyx fractures, had a significantly higher risk of ARDS than did patients without CVF.
Comparisons of incidence, and HR of outcome by subtypes of clinical vertebral fracture
Figure 2A–2D show that the CVF cohort had a significantly higher cumulative proportion of AD (p=0.02; figure 2A), CHF (p<0.001; figure 2B), pneumonia (p<0.001; figure 2C) and ARDS (p<0.001; figure 2D) than did the no-CVF cohort.
Cummulative incidence of aortic dissection (A), congestive heart failure (B), pneumonia (C) and acute respiratory distress syndrome (D) in patients with clinical vertebral fracture and comparison patients
Discussion
To the best of our knowledge, this is the first population-based, longitudinal cohort study to focus on the correlation between CVF and the subsequent risks of specific cardiopulmonary diseases. The main results demonstrated that CVF is significantly associated with an increased risk of several specific cardiopulmonary diseases, including AD, CHF, pneumonia and ARDS.
Demographics and comorbidity
In our study, patients older than 65 years and females accounted for the majority of participants. In fact, the incidence and prevalence of vulnerable fractures, accompanied with population ageing and subsequent frequently occurring home accidents, are steadily rising.38 In addition, CVF in women is constantly a consequence of postmenopausal bone loss.5 7 8 According to recent studies, the prevalence of women older than 50 years who experienced at least one CVF event was 23%–26%, which was higher than that of men (21.5%).39 40 It is noteworthy that young adults aged ≤49, though represented the minority of CVF patients, bore a significant heightened risk of developing adverse outcomes in the following analyses. We speculate that CVF in young adults could have more prominent influence on the outcome diseases without the interaction of multiple potential comorbidities and unknown confounders. Another explanation is that CVF is less frequent in a young, healthy population; it could be more severe and detrimental, strengthening the correlations between the investigated diseases.
Clinical vertebral fracture and aortic dissection
In our analysis, with or without CVF, the incidence of AD was higher in men, elderly patients older than 65 years, and those with coexisting comorbidities; this finding is in line with previous epidemiological investigations.11 12 41 Moreover, compared with patients without CVF, CVF patients, especially female patients, younger population (age ≤49) and those without comorbidities, bore a higher risk of subsequent AD development. Studies that have focused on this correlation are scarce. Interestingly, prior studies have provided evidence for the strong correlation between poor bone health with major fragility fracture and abdominal aortic calcifications.42 43 With the progressive destruction of intima-media layer accompanied with new bone-like tissue deposition in the aortic wall, aneurysm or dissection might tend to occur. Other potential explanations we suppose include the intractable pain induced by fractures, accompanied with increments in sympathetic tone, stress, hypertension and the impact on the vascular wall, as well as an unfavourable sedentary life style could all contribute to the formation of AD.
Clinical vertebral fracture and congestive heart failure
Our study indicated one counterintuitive result that women bore a higher overall incidence of CHF than men did. However, previous investigations of sex-specific epidemiology of CHF have demonstrated that women with atrial fibrillation have a higher incidence of heart failure with preserved ejection fraction, especially in very old age compared with men.44–46 In this study, CVF was associated with an increased risk of CHF, and the results remained statistically significant across various age and sex strata, as well as with or without comorbidities. In a cross-sectional analysis, Lyons et al 47 demonstrated that more than one-tenth of heart failure patients had radiologic recognisable vertebral fracture, and among those, multiple vertebral fractures accounted for one half, indicating the close correlation between these two diseases. Moreover, Sennerby et al 48 conducted a twin population study and proposed that specific genes involved in cellular mechanisms that shared by the vasculature and bone might connect the close relationship between cardiovascular diseases and fractures. Additionally, the most common aetiology of CVF, osteoporosis, together with CHF, share common risk factors and etiologic mechanisms, including advantaged age, female sex, hypovitaminosis D, renal insufficiency, diabetes, a smoking habit, activation of the renin-angiotensin-aldosterone system, hypersecretion of parathyroid hormones and oxidative/nitrosative stress.23 47 49–52 In a meta-analysis, Veronese et al 53 concluded that alterations in signalling pathways of bone remodelling and arterial calcifications could contributed to the higher cardiovascular risk. Indeed, diffuse vascular calcifications accompanied with bone loss could result in a higher afterload on the left ventricle, leading to subsequent left ventricular hypertrophy and finally, congestive heart failure.42 43 Furthermore, unfavourable outcomes following fracture, including a loss of functional and social activities, dependency with poor quality of life, higher serum cortisol levels accompanied with depressive disorder, higher inflammatory markers, lower drug and diet compliance, a sedentary life style and arrhythmia or cardiac ischaemic events caused by high sympathetic activity, might all contribute to the deterioration of heart function.50 54
Clinical vertebral fracture and pneumonia, acute respiratory distress syndrome and subtypes analysis
Our study results reveal that patients with CVF bore a significantly heightened risk of subsequent pneumonia and ARDS across all strata of age and sex and irrespective of the presence of comorbidities. Further analyses demonstrated the strongest correlation between cervical CVF combined with SCI and risks of pneumonia and ARDS. In a 2 year retrospective multicenter trauma registry analysis, Fletcher et al 55 noted that 16% of elderly patients older than 65 years with cervical spine trauma ultimately developed pneumonia. Other studies have revealed the incidence of pulmonary complications following cervical spine trauma to be 35%–95%,56 57 and among these complications, the most common type was pneumonia and atelectasis, although ARDS was the most severe type.58–60 There are several possible explanations. First, deformity of the vertebral body or even kyphosis might decrease the lung capacity and therefore impair the pulmonary function. Prior studies have indicated that a single vertebral fracture would decrease the predicted forced vital capacity by 9%, increase the risk of restrictive lung disease.1 2 61 Harrison et al 62 conducted a systemic review of 4 case-control studies and reported that women with osteoporotic vertebral fractures or kyphosis were associated with decreased predicted vital capacity, as well as total lung capacity. Furthermore, Krege et al 63 estimated that spine fracture burden is linked with restrictive, but not obstructive lung disease. The authors further concluded that patients with marginally compensated pulmonary function may not tolerate the superimposed lung restrictive change resulting from vertebral fractures and thus, leading to a further compromised pulmonary function and subsequent lung diseases. Second, cervical CVF combined with SCI might cause paralysis of the diaphragm and hypoactivity of the respiratory accessory muscles, which results in hypoventilation. In addition, the imbalance of sympathetic-parasympathetic interactions would result in an elevated airway tone, bronchorrhea and poor clearance, which are all associated with the development of various pulmonary complications.64 65 Third, patients with SCI are prone to develop aspiration and subsequent pulmonary infection due to impaired neuromuscular transmission. Finally, similar to rib fractures, worsening pain related to CVF might impair cough and secretion clearance, leading to atelectasis and subsequent lung infection.24
Limitations
The major strength of our study is sampling from a large nationwide database, which covers nearly 100% of all residents in Taiwan, and stable outcomes could be achieved with such adequate, representative samples. However, the inevitable limitations should be discussed. First, all disease definitions and sample selection in our study were based on the ICD-9-CM coding, which has been rigorously scrutinised and peer-reviewed by clinical physicians, the declaration unit of medical institutions and finally the NHI administration. However, miscoding or misclassification might still exist, although it is considered rare. Similarly, diagnostic criteria applied, as well as physician’s ability to diagnose the investigated diseases might vary among different hospitals and areas. Second, retrospective dataset analysis results cannot be used to determine causal relationships. Third, several crucial variables could not be obtained from our dataset, including family history, education and socioeconomic status, information of life style and physical activity, body weight, smoking habits, disease severity, laboratory results, radiologic reports and estimated pain scores, which are potential confounders that might have affected the results. Fourth, a considerable portion of vertebral fracture patients with slight or no symptoms might not have been diagnosed or might have even been overlooked in clinical settings; thus, the true incidence of CVF and the inferred association between CVF and cardiopulmonary diseases could be underestimated. Fifth, patients with CVF might have one or more overlapping etiologies include osteoporosis, trauma and malignancies, etc. Therefore, it was technically infeasible to simply divide the CVF patients into several subgroups for sub-analysis based on the coding of etiologies. Sixth, our sampled participants were retrieved from NHIRD from January 1, 2000, to December 31, 2010. Ageing property of the data might not truly reflect the current medical conditions. Finally, because of geographic and epidemiologic discrepancies, our results might not be applicable to other countries or regions.
Conclusion
In conclusion, our study results support the hypothesis that CVF is associated with subsequent risks of AD, CHF, pneumonia and ARDS. Future studies are warranted to delineate the actual pathophysiologic mechanisms underlying this correlation and to develop optimal strategies for reducing the heath care burden of CVF and its complications. Based on our results, we suggest that patients with CVF should be targeted for further screening and preventive interventions for cardiopulmonary diseases.
References
Footnotes
T-YY and C-YL contributed equally.
Contributors The authors’ individual contributions are outlined as follows. Conception and design: F-YL. and T-YY. Administrative support: T-YY. Data collection and organization: F-YL, W-KC, C-CL, C-HK, T-YY & C-YL. Data analysis and interpretation: F-YL, W-KC, C-CL, C-HK, T-YY & C-YL. Manuscript writing: F-YL, W-KC, C-CL, C-HK, T-YY & C-YL. Final approval of the manuscript: F-YL, W-KC, C-CL, C-HK, T-YY & C-YL.
Funding This work was supported by grants from the Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW108-TDU-B-212-133004); China Medical University Hospital (DMR-107-125); Academia Sinica Stroke Biosignature Project (BM10701010021); MOST Clinical Trial Consortium for Stroke (MOST 108-2321-B-039-003-); Tseng-Lien Lin Foundation, Taichung, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; The Department of Medical Research at Mackay Memorial Hospital (MMH105-87; MMH-106-81; MMH-107-71; MMH-107-102; MMH-107-135).
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval This study was approved by the Ethics Review Board of China Medical University Hospital, Taiwan (CMUH-104-REC2-115-CR4). The IRB waived the consent requirement.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data may be obtained from a third party and are not publicly available.