Article Text
Abstract
Introduction A majority of patients who receive myeloablative therapy prior to hematopoetic stem cell transplantation develop oral mucositis (OM). This adverse cytotoxic effect manifests as oral mucosal erythema and ulcerations and frequently necessitates high doses of morphine for pain alleviation. OM may also interfere with food intake and result in parenteral nutrition, weight loss and impaired quality of life. To date, there have been a few studies of evidence-based interventions for prevention of OM. Cooling the oral mucosa using ice chips in conjunction with chemotherapy is known to reduce the severity of OM although clinical application is still limited due to several disadvantages. The primary endpoint of this study is therefore to evaluate the efficacy of an innovative intraoral cooling device (Cooral) compared with ice cooling in reducing the degree of OM, in patients with myeloma or lymphoma.
Method and analysis A total of 180 patients from four different university hospitals in Sweden will be randomised to ice or Cooral in a proportion of 1:1. The degree of OM will be assessed at eight intraoral locations, in accordance with the Oral Mucositis Assessment Scale and WHO scale. Patients will be registered beginning at admission and will continue until discharge or until day +28. The primary variable is analysed in a multiple linear regression model. The significance level used is 5%.
Ethics and dissemination The study protocol, questionnaire, diaries and letter of invitation to participants have been reviewed by the local ethical board in Göteborg. The trial results will be published in a peer-reviewed journal and disseminated to participants.
Trial registration number NCT03203733; Pre-results.
Protocol version Version 4, 2017-06-05
- chemotherapy
- myeloma
- lymphoma
- bone marrow transplantation
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of the study
Prospective randomised multicentre study with 180 patients evaluating the efficacy and tolerability of two cooling methods.
Blinded assessment of oral mucositis by dentists specialised in orofacial medicine and pathology.
Longitudinal study lacking an untreated control group.
Patients younger than 16 years of age are not included in this study.
Introduction
Background and rationale
Hematopoietic stem cell transplantation (HSCT) is successfully used for a number of malignant blood diseases. In autologous stem cell transplantation (ASCT), the patient’s own blood stem cells are used in order to preserve bone marrow function after administration of high doses of cytostatics.
Chemotherapy has many side effects, one of which is oral mucositis (OM). OM is a lesion which involves the mucous membrane of the oral cavity and affects up to 80% of patients who receive high doses of cytostatics in preparation for HSCT.1 2 The lesion of the oral mucosa manifests itself as painful erythema and ulcerations 3 and may require high doses of morphine for pain alleviation.4 Furthermore, OM may interfere with food intake, which can lead to undernourishment, weight loss and impaired quality of life.5
Today, there are few treatment methods intended to prevent OM. An extensive literature search shows that the best-documented preventive method is cooling of the oral mucous membrane with ice, before, during and after chemotherapy.6
Despite well-substantiated documentation, there is limited use of ice cooling as a method to prevent OM in clinical practice. As ice can give rise to shooting pains in the teeth and other discomfort for the patient, this may lead to lower cooperation. In addition, it is important that the ice is made from water of good quality to minimise the risk of infections.
To prevent OM, an intraoral cooling device (Cooral) has been developed.7 Cooral consists of closed conduits with continuously circulating water, to cool the cheeks, lips, floor of the mouth, tongue gums and hard palate. By offering Cooral, we intend to prevent OM.
Therefore it’s of interest to conduct a randomised controlled study to evaluate Cooral for prevention of OM.
Specific objectives or hypotheses
The objectives are to compare Cooral and ice cooling with regards to efficacy and tolerability.
Trial design
An open randomised controlled trial with blinded evaluation of OM.
Methods: participants, interventions and outcomes
Study setting
Patients with myeloma or lymphoma at Karolinska University Hospital (figures 1 and 2), and Uppsala University Hospital (figures 3 and 4), and patients with myeloma at the University Hospitals in Linköping and Örebro (figure 3) who are to undergo ASCT will be asked to participate in the study.
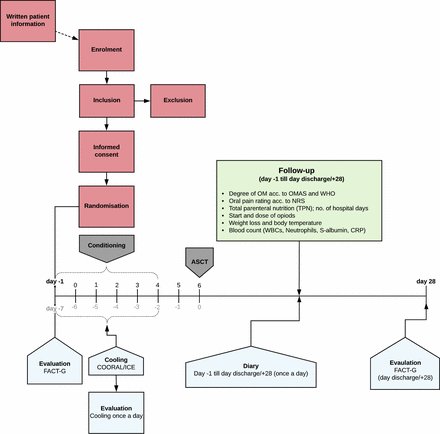
Flowchart for patients with myeloma at Karolinska University Hospital. Day 0: admission, chemotherapy conditioning, oral mucosal cooling along with completion of quality of life (QoL) questionnaire (FACT-G) and evaluation of cooling method. Day 1: autologous stem cell transplantation (ASCT). Follow-up (green box) and perception of oral problems using a diary begins at admission and continues until discharge or day +28. QoL (FACT-G) is evaluated again at discharge. CRP, C reactive protein; NRS, Numeric Pain Rating Scale; OM, oral mucositis; OMAS, Oral Mucositis Assessment Scale; WBC, white blood cell.
Flowchart for patients with lymphoma at Karolinska University Hospital. Day −1: admission, along with completion of quality of life (QoL) questionnaire (FACT-G). Day −1 to 4: chemotherapy conditioning, oral mucosal cooling along with completion of evaluation of cooling method. Day 5: recovery. Day 6: autologous stem cell transplantation (ASCT). Follow-up (green box) and perception of oral problems using a diary begins at admission and continues until discharge or day +28. QoL (FACT-G) is evaluated again at discharge. Days highlighted in black indicates the timescale used in this study while grey is routinely used at the hospital. CRP, C reactive protein; NRS, Numeric Pain Rating Scale; OM, oral mucositis; OMAS, Oral Mucositis Assessment Scale; WBC, white blood cell.
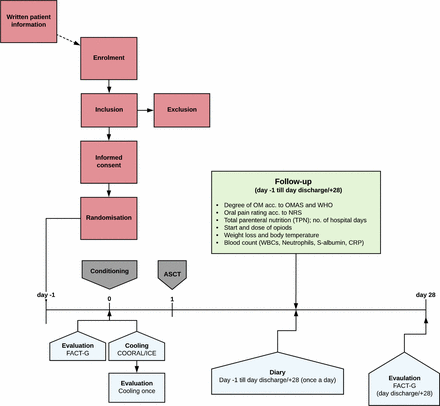
Flowchart for patients with myeloma at Uppsala University Hospital and University Hospitals in Linköping and Örebro. Day –1: admission. Day 0: chemotherapy conditioning, oral mucosal cooling along with completion of quality of life (QoL) questionnaire (FACT-G) and evaluation of cooling method. Day 1: autologous stem cell transplantation (ASCT). Follow-up (green box) and perception of oral problems using a diary begins at admission and continues until discharge or day +28. QoL (FACT-G) is evaluated again at discharge. CRP, C reactive protein; NRS, Numeric Pain Rating Scale; OM, oral mucositis; OMAS, Oral Mucositis Assessment Scale; WBC, white blood cell.
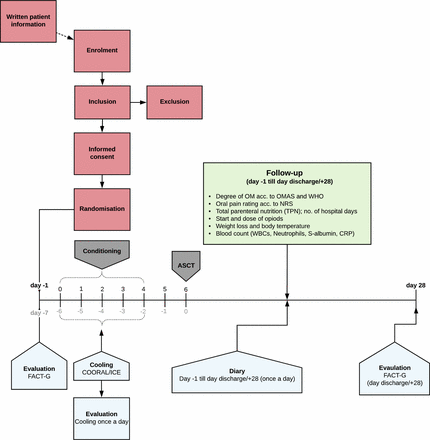
Flowchart for patients with lymphoma at Uppsala University Hospital. Day −1: admission, along with completion of quality of life (QoL) questionnaire (FACT-G). Day 0 to 4: chemotherapy conditioning, oral mucosal cooling along with completion of evaluation of cooling method. Day 5: recovery. Day 6: autologous stem cell transplantation (ASCT). Follow-up (green box) and perception of oral problems using a diary begins at admission and continues until discharge or day +28. QoL (FACT-G) is evaluated again at discharge. Days highlighted in black indicates the timescale used in this study while grey is routinely used at the hospital. CRP, C reactive protein; NRS, Numeric Pain Rating Scale; OM, oral mucositis; OMAS, Oral Mucositis Assessment Scale; WBC, white blood cell.
Eligibility criteria
Inclusion criteria
Patients aged 16 or over diagnosed with myeloma or lymphoma.
Able to communicate in Swedish.
Treated with melphalan (myeloma), BEAM/BEAC (lymphoma), before ASCT.
Exclusion criteria
Patients who do not understand oral and written information in Swedish.
The patient is taking part in another study which, in the doctor’s judgement, can affect the result of this study.
The patient is receiving post-treatment care at a different hospital than where the stem cell transplant took place and follow-up is not possible.
The doctor judges that the patient is for some reason not suitable for the study.
Interventions
Ice
Patients will be provided with ice cubes/crushed ice or ice pop 30 min prior to the start of chemotherapy. Once the ice melts, the liquid is rinsed around the mouth to cool as large surface as possible of the oral mucosa. In addition, to achieve cooling of the hindmost part of the throat, the liquid is gurgled for a few seconds before it is swallowed or spat out. The procedure is repeated until 30 min after the termination of the cytostatic infusion. During treatment the patient may if necessary rest for a maximum of 5 min. Food and drink should, whenever possible, be consumed either before or after the cooling session. Cooling continues throughout conditioning with cytostatics in the treatment schema melphalan (myeloma) and BEAM/BEAC (lymphoma). In lymphoma conditioning cures with a 12-hour infusion time (e.g., cytarabine), the cooling starts initially 30 min prior to the start of cytostatic treatment and continues 30 min after the start of the 12-hour cytostatic infusion. Then the patient is provided with ice cubes/crushed ice or ice pop for 30 min every 4 hours during the infusion. It is important to end with 30 min cooling of the oral mucous membrane after each completed cytostatic administration.
Cooral
Prior to treatment, the patient receives clear oral instructions and a demonstration on the use of Cooral by the nurse responsible for the patient. When the patient him/herself is able to administer the intraoral component until it feels comfortable, the responsible nurse will check to ensure that it has good contact with the oral mucous membrane. Cooling begins 30 min before the start of chemotherapy and continues until 30 min after the termination of the cytostatic infusion. During treatment, the patient may if necessary take out the component, for a maximum of 5 min, and replace it again. Food and drink should, whenever possible, be consumed either before or after the cooling session. Cooling continues throughout conditioning with cytostatics in the treatment schema melphalan (myeloma) and BEAM/BEAC (lymphoma). In lymphoma conditioning cures with a 12-hour infusion time (e.g., cytarabine), the cooling starts initially 30 min before the start of cytostatic treatment and continues 30 min after the start of the 12-hour cytostatic infusion. Then the patient is provided with Cooral for 30 min every 4 hours during the infusion. It is important to end with 30 min cooling of the oral mucous membrane after each completed cytostatic administration.
Outcomes
The primary objective is to study patients with myeloma or lymphoma undergoing ASCT, to evaluate whether cooling with Cooral compared with ice cubes/crushed ice or ice pop succeeds in reducing the degree of OM according to the Oral Mucositis Assessment Scale (OMAS total).
The secondary objectives are to evaluate OMAS total divided according to OMAS ulceration, OMAS erythema, degree of OM according to WHO, tolerability of either cooling method, subjective experience of OM, rating of general quality of life and oral pain, number of days with total parenteral nutrition (TPN), number of hospital days, total dose of opioids and C reactive protein (CRP) during time in care.
The tertiary objectives are to evaluate weight loss, leucocyte plasma concentration (LPC), number of days until bone marrow response, S-albumin and body temperature.
Participant timeline
Total cooling time for myeloma 1.5 hours and lymphoma 3–6 hours. All patients are followed beginning at admission and will continue until discharge or until day +28.
Sample size
A sample size of 90 patients per group will give a power of 80% to discover an average difference of at least 0.42 OMAS units.8 The analysis is based on the SD for OMAS being 1 in both groups, and the use of an independent t-test with a significance level of 5%.
Recruitment
All patients with myeloma or lymphoma at Karolinska University Hospital and Uppsala University Hospital, and patients with myeloma at the University Hospitals in Linköping and Örebro who are to undergo ASCT will be asked to participate in the study. Information will be given at the time of stem cell apheresis and in material sent to the patient in connection with the invitation letter, with information about admission to the ward for ASCT. Inclusion in the study will take place after written consent on arrival at the ward to be admitted for ASCT. For underage patients (16–17 years), parents will also be informed and asked if they consent to their children’s participation. Estimated time for inclusion is approximately 1.5 years starting from 12th June 2017.
A total of 180 patients will be recruited and randomised to ice or Cooral in a proportion of 1:1. Expected number of patients: from Karolinska University Hospital (70), Uppsala University Hospital (80), Linköping University Hospital (15) and Örebro University Hospital (15).
Patient and public involvement
Comparison of the balance between efficacy and safety for Cooral and ice in the prevention of OM is in the best interest of patients. Patients were not involved in the design of this study or in the recruitment or conduct of the study. The study results will be disseminated to study participants orally and in writing. The burden of the interventions will be assessed by the patients themselves. No patient advisers were involved in the design or conduct of the study.
Methods: assignment of interventions
Sequence generation
Randomisation will be managed centrally by the study administration in connection with admission to treatment. Each hospital will be given randomisation lists to follow. Randomisation will be stratified with regard to department and diagnosis.
Allocation concealment mechanism
The patients will undergo balanced randomisation with randomly varying block sizes (two, four or six patients) where one, two or three experiments are distributed in sequences and one, two or three controls. A block can be, for example, ‘ce’ if there are two patients, and ‘ceec’ and ‘eeccec’ if there are four or six patients, respectively. Each hospital is blinded to the size of the blocks.
Implementation
Participants will be assigned by the responsible healthcare providers.
Blinding (masking)
The dental staff in charge of assessing OM and the statistician will be blinded to the interventions.
Data collection, management and analysis will be done by Karolinska Trial Alliance (KTA) and Uppsala Clinical Research (UCR), two independent contract research organisations.
Methods: data collection, management and analysis
Data collection methods
All measurements, with the exception of the patient’s subjective assessment of the cooling method, will be registered beginning at admission and will continue until discharge or until day +28. Cytostatic infusion generally starts on the day after admission. Grading of OM according to OMAS and WHO is done three times a week, for example, Monday, Wednesday and Friday, by a dentist/dental hygienist. For each day the patient is hospitalised, assessment with WHO is also performed by nurses/assistant nurses who are not blinded to the treatment group. Clinical routines differ between study centres and therefore the number of assessments can be lower during the week of admission, depending on when patients are admitted.
The degree of OM is assessed at eight intraoral locations, in accordance with the OMAS (graded 0–3 for ulceration and 0–2 for erythema). Zero corresponds to ‘normal’ while 3 and 2 are ‘sore >3 cm2’ and ‘severe erythema’, respectively. The assessment generates both an average for OMAS ulceration (0–3) and OMAS erythema (0–2) and a total average OMAS (0–5), which is the mean of both ulceration and erythema.
Besides OMAS, ulceration and erythema are also assessed with the WHO scale (graded 0–4) where 0 is ‘no mucositis’ and 4 is ‘ulceration, total parenteral nutrition’.
Prior to myeloablative therapy, all patients at each of the four study sites undergo a complete oral/dental examination by a dentist specialised in orofacial medicine and pathology followed by an odontological decontamination. Meticulous information and instructions for oral hygiene maintenance is also received. Patients who develop OM are initially treated with paracetamol or opioids, depending on degree of symptoms, and further assisted by healthcare professionals to maintain a good oral hygiene. In more severe cases, a dentist or a dental hygienist is contacted for assistance and further support with oral care maintenance.
The dental staff responsible for OM assessment were calibrated prior to study start. ICC coefficient: OMAS=0.94 (excellent); WHO=0.67 (good). Following inclusion of 100 patients a second ICC will be performed to assess inter- and intra-rater reliability. All other staff involved in the study have undergone a solid education in assessing oral health status according to WHO.
Rating of pain is done with the Numeric Pain Rating Scale (NRS) one to two times daily if the patient is kept on the ward. Alternatively, it is done every other day according to the respective department’s routine for outpatient care if the patient is at home or in a home-like environment. In the case of outpatients, the subjective rating of pain is noted in the patient documentation through daily telephone contact with the responsible nurse. Body temperature is registered in the oral cavity, ear or axillary region in accordance with the department’s routines.
Furthermore, the patients, after cooling ends, assess the tolerability of the respective cooling method with the aid of a questionnaire (online supplementary file 1) developed for the study. The questionnaire is intended to give some idea of any discomfort or side effects the patients feel as a result of the cooling method.
Supplementary file 1
The patients assess their perception of oral problems daily with the aid of specific questions in a diary (online supplementary file 2) developed for the study. The questions are intended to give a picture of the effect of OM on the patient’s general status.
Supplementary file 2
General quality of life is assessed twice during the study period, before the start of treatment and at discharge, with a validated quality of life instrument (online supplementary file 3).
Supplementary file 3
Information about TPN, number of hospital days, total dose of opioids, weight loss and body temperature will be retrieved from patient records. Laboratory results of blood tests will be retrieved from each department’s register of test results.
The result of the assessments is documented on special CRF (case report forms) for the purpose, referred to in the study as ‘checklists’.
Participant retention
Complete follow-up of patients who discontinue cooling prematurely or otherwise deviate from the intervention protocol.
Data management
Data will be collected on paper-based checklist created by the sponsor. Each checklist will be identified by a pre-printed trial number and a combination of patient study number (assigned at registration). The investigator or an authorised staff member will complete the checklist on site. The checklist must be dated and signed by the investigator on completion.
The PheedIt system will be used for data capture of clinical data and this will serve as the clinical database for the study. UCR will be responsible for set-up of the clinical database, support, programming of logical computerised checks, data management plan and management of the PheedIt system. Data from the checklist will be entered, cleaned and validated by the sponsor-appointed person. This appointed person should have signed a secrecy agreement with each study site and have the task delegated to enter/edit data into the database. The completed patient questionnaires (online supplementary files 1–3) will also be entered into the clinical database by the sponsor-appointed person. The entered data will be subject to logical computerised checks, and the output from these checks will be sent to the sponsor-appointed person for review and action. Actions to be taken by study staff as a result of the review of the check output should be documented, for example, on a data clarification form (DCF). The DCF must be signed and dated by the investigator, thereafter the clinical database can be updated. Any corrections made to entered data will be audited.
The original checklists and questionnaires are source data and will be kept on site. Copies of the checklists and questionnaires will be collected by the sponsor-appointed person.
Statistical methods
All analyses are at population level: intention to treat.
Analysis of the primary variable
The primary endpoint is peak OMAS (total), that is, the highest measured OMAS total during the time in care. The primary variable is studied in a multiple linear regression model. Fixed explanatory variables are treatment group, type of cancer and centre. An initial model also includes interaction between treatment and type of cancer and interaction between treatment and centre. If the interaction effects are not significant, these are excluded from the final model. The significance level used is 5%.
Analyses of the secondary variables
OMAS ulceration and OMAS erythema indices, analysed in the same way as peak OMAS (total), that is, peak value, is used as a target variable in a multiple regression model. The same explanatory variables are used as in the final model for peak OMAS (total).
Incidence of OM (grades 1–4 according to WHO) and incidence of severe OM (grades 3–4 according to WHO) are analysed with the aid of logistic regression with the same explanatory variables as in the final model for peak OMAS (total). Significance level is 5%.
Tolerability. Incidence of problems (grades 1–3) and severe problems (grades 4–7) are analysed in the same way as the incidence of OM and severe OM. Significance level is 5%.
Subjective ratings of OM, general quality of life and oral pain are analysed non-parametrically, above all with the help of Mann-Whitney U test.
Quantitative data such as number of days with TPN, number of hospital days, total dose of opioids and CRP are analysed with independent t-test or with Mann-Whitney U test if the observed data material shows a significant skew. Descriptive statistics and explorative analysis will be used to study any differences between centres.
Analysis of the tertiary variables
Weight loss, LPC, number of days until bone marrow response, S-albumin and body temperature are analysed with independent t-test or with Mann-Whitney U test if the observed data material shows a significant skew. Descriptive statistics and explorative analysis will be used to study any differences between centres.
Additional analyses
Separate analyses will be conducted with regard to study site and with regard to diagnosis (myeloma/lymphoma).
Missing data
OMAS (total) and WHO performed by dentists/dental hygienist after treatment are replaced with WHO performed by nurses. WHO replacing OMAS (total) will be translated into OMAS (total).9 In the final analysis, the dentist assessment is primarily used. For OMAS subindex, the highest value is used as the peak OMAS subindex if at least one OMAS subindex is available after treatment. If there is no OMAS subindex after treatment, the patient’s baseline is used as peak OMAS subindex.
For other secondary/tertiary variables, the strategy is to use the mean value of the preceding and following value. If the preceding value is missing, the following value is used. If the following value is missing, the technique used is last value carried forward.
Methods: monitoring
Data monitoring
To protect the patients’ safety during the trial, the results will be monitored by a Data Monitoring Committee (DMC), consisting of an experienced biostatistician and a clinician with long experience of clinical trials. Both are independent of the sponsor and will provide an impartial recommendation for the continuation of the study. Separate working instructions will be provided as a ‘charter’ to the DMC. A conservative stopping rule according to the O’Brien-Fleming boundary will be applied to minimise the effect of the interim analysis on the statistical strength at the end of the trial.
Interim analysis
The DMC will have two members, an experienced biostatistician and a clinician with considerable experience of clinical trials. The CRO will provide the DMC with a first interim analysis when 100 patients have been treated. Based on the results, the DMC will recommend the sponsor to continue or terminate the trial without communicating any results. The DMC will decide about further interim analyses. Before the DMC recommends early termination of the trial, it will try to get advice from the Food and Drug Administration.
Harms
Adverse events related to the Cooral/ice treatment sessions will be assessed by the questions addressed in the patients’ questionnaires ‘Cooral cooling’, ‘Cooling with ice/crushed ice/ice pop’. The adverse events will be summarised and included in the final rapport of the clinical study. Serious adverse events that are related to the Cooral/ice treatment sessions will be reported to all sites and principal investigators will be informed/updated. Any errors of the medical device will be documented and taken care of/repaired by the sponsor.
Auditing
The study will be monitored by KTA to ensure that it is carried out in accordance with the established study protocol, Helsinki declaration, ISO14155:2011 and other applicable guidelines and regulations. A monitoring plan has been established.
Ethics and dissemination
Protocol amendments
Important protocol modifications will be communicated by KTA to investigators, trial participants, trial registries, review board, journals and regulators.
Consent or assent
Written informed consent (online supplementary file 4) will be obtained from all patients included in the study.
Supplementary file 4
Confidentiality
Patients will have patient study number, which is linked to their identity for traceability. The study number is used for all documents to ensure that the patient identity is not disclosed. Only the healthcare staff in charge at the clinic have access to journals and ‘Subject Enrolment and Identification Log’ where the patient’s identity appears. All data will be confidential and password protected throughout the study. Patient identity is protected in the final report and on publication of the study.
Access to data
KTA and UCR, authors and investigators.
Ancillary and post-trial care
Usual care according to the clinical standard. Participants are insured by QBE and Chubb insurances. Compensation will be given to those who suffer harm from trial participation.
Dissemination policy
The plan for the investigators and sponsors is to publish a full scientific article in a peer-reviewed journal. The sponsor has no intention to use professional writers. Public access to the full protocol, participant-level dataset and statistical code will be provided on request.
Acknowledgments
The authors would like to thank Sajjad Saffari, BMSc, for contributing with the design of the flowcharts.
Footnotes
Contributors JW was involved in initial study conception, trial design, intervention development, protocol and manuscript preparation, and ethics application. AS was involved in trial design and input to protocol preparation. MG was involved in statistical advice, input to trial design and protocol preparation. All authors have fulfilled the ICMJE criteria for authorship.
Funding This work was supported by BrainCool AB, VINNOVA, grant number 2016-04171 and Gothenburg Dental Society, grant number 2017-12-21.
Competing interests JW is currently in receipt of a PhD scholarship funded by BrainCool AB. AS is employed part-time at BrainCool AB.
Ethics approval Regional review board in Gothenburg (Dnr: 586-15).
Provenance and peer review Not commissioned; externally peer reviewed.
Patient consent for publication Not required.