Article Text
Abstract
Introduction Clostridium difficile infections (CDI) are common, costly and potentially life threatening. Most CDI will respond to antibiotic therapy, but 3%–10% of all patients with CDI will progress to a severe, life-threatening course. Complete removal of the large bowel is indicated for severe CDI. However, the 30-day mortality following surgical intervention for severe CDI ranges from 20% to 70%. A less invasive approach using surgical faecal diversion and direct colonic lavage with polyethylene glycol (PEG) and vancomycin has demonstrated a relative mortality reduction of approximately 50%. As an alternative to these operative approaches, we propose to treat patients with bedside intestinal lavage with PEG and vancomycin instillation via nasojejunal tube, in addition to usual antibiotic management. Preliminary data collected by our research group are encouraging.
Methods and analysis We will conduct a 1-year, single-centre, pilot randomised controlled trial to study this new treatment strategy for patients with severe CDI and additional risk factors for fulminant or complicated infection. After informed consent, patients with severe-complicated CDI without immediate indication for surgery will be randomised to either usual antibiotic treatment or usual antibiotic treatment with the addition of 8 L of PEG lavage via nasojejunal tube. This pilot trial will evaluate our eligibility and enrolment rate, protocol compliance and adverse event rates and provide further data to inform a more robust sample size calculation and protocol modifications for a definitive multicentre trial design. Based on historical data, we anticipate enrolling approximately 24 patients during the 1-year pilot study period.
As a pilot study, data will be reported in aggregate. Between-group differences will be assessed in a blinded fashion for evidence of harm, and to further refine our sample size calculation.
Ethics and dissemination This study protocol has been reviewed and approved by our local institutional review board. Results of the pilot trial and subsequent main trial will be submitted for publication in a peer-reviewed journal.
Trial registration number NCT02466698; Pre-results.
- Gastrointestinal infections
- Adult intensive and critical care
- Clostridium difficile
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Strengths and limitations of this study
The ERASE trial is a 1-year, parallel group, pilot randomised clinical trial (RCT) of intestinal polyethylene glycol (PEG) lavage in addition to usual antibiotic treatment versus usual antibiotic treatment alone. This is the first RCT assessing this novel, non-operative approach to the management of severe-complicated Clostridium difficile infection.
The study follows a pragmatic design and uses broad inclusion criteria to satisfy the definition of ‘severe-complicated’ C. difficile infection, supporting external validity.
This is a feasibility trial and is not powered to determine treatment effectiveness.
Results of the ERASE pilot will inform the design and conduct of a future multicentre RCT of intestinal PEG lavage to improve mortality outcomes for patients with severe-complicated C. difficile infections.
Introduction
Background and rationale
Clostridium difficile is a gram-positive, anaerobic spore-forming bacterium first identified as the cause of antibiotic-associated diarrhoea in the 1970s.1 Commensal gut flora acts as a barrier to C. difficile colonisation, but can be disrupted by antibiotic therapy. During overgrowth of toxigenic strains, potent exotoxins are produced that bind to intestinal epithelial cells, thereby inducing inflammation, mucopurulent secretions and damage to mucosal structures.2 Clostridium difficile infection (CDI) can span a range of clinical presentations from an asymptomatic carrier state to life-threatening infection. C. difficile colitis manifests with high-volume watery diarrhoea and abdominal pain, and is associated with evidence of systemic inflammatory response with fever and leukocytosis. In the USA, the incidence of CDI doubled from 5.5 cases per 10 000 population in the year 2000 to 11.2 cases per 10 000 in the year 2005.3 Fulminant C. difficile colitis occurs in approximately 3% of CDIs4 and is associated with significant complications such as perforation, megacolon, ileus and death. CDI has been identified as a direct cause of death in 1%–2% of affected patients,4 but this incidence has increased in recent years. Emergency surgery is required in approximately 1% of all CDI, carrying a mortality rate of approximately 40%.5
The Society for Healthcare Epidemiology of America and the Infectious Diseases Society of America (IDSA)6 guidelines define severe CDI as leukocytosis with a white blood cell (WBC) count of greater than 15 000 cells/µL or a serum creatinine level 50% above the premorbid baseline. Enteral vancomycin, 125 mg four times daily, for 10–14 days is recommended. Severe-complicated disease is suggested by associated complications including hypotension, shock, ileus or megacolon. High-dose enteral vancomycin regimen (500 mg every 6 hours) combined with intravenous (IV) metronidazole (500 mg every 8 hours) for 10–14 days is suggested, and surgery is to be considered. Given the significant associated postoperative mortality, operative intervention prior to serum lactate rising above 5 mmol/L is suggested.6
The role of faecal microbiota transplant (FMT) in the treatment of CDI is evolving. The use of FMT in the treatment of recurrent CDI has been established in the literature with several randomised controlled trials showing superiority over antibiotic therapy.7 Although FMT for the treatment of severe and severe-complicated CDI has shown promise in early case series, a recent systematic review found that there is currently insufficient evidence to recommend the use of FMT in this population.7 8
Despite a lack of definitive evidence, total abdominal colectomy (TAC) is currently recommended as the surgical intervention of choice in the setting of severe-complicated CDI.6 9 10 However, 30-day mortality following total colectomy and end ileostomy for fulminant CDI is 19%–71%.5 Given the high mortality of fulminant CDI, early surgical intervention is recommended.9 Furthermore, data regarding the optimal timing for surgical intervention are also lacking. The Eastern Association for Surgery on Trauma guideline9 strongly recommends early surgical management, defined as being before the development of shock or the requirement for vasopressors, based on very low-quality evidence, but with the potential for significant mortality reduction (relative risk (RR) of 0.50; 95% CI 0.35 to 0.72). Despite these potential benefits, anticipating which patients will develop severe-complicated CDI mandating surgery is often unclear early in the disease process. Indeed, only approximately 30% of patients with severe CDI go on to emergent surgical intervention,5 suggesting a significant risk of overtreatment with an early surgical approach. Numerous prediction scores have been developed, but none have been well validated.11–14 Furthermore, TAC is a significant surgical insult and carries with it significant morbidity. Therefore, surgical intervention is often delayed until refractory severe-complicated CDI is clearly established, at which point the optimal window of early surgical intervention may have been missed.
Less morbid surgical treatments have been suggested. Neal et al 14 demonstrated that surgical diversion of the faecal stream with a loop ileostomy and intraoperative antegrade colonic lavage may effectively treat severe CDI. Widely referred to as the ‘Pittsburgh Protocol,’ the regimen includes colonic lavage with 8 L of polyethylene glycol (PEG 3350) intraoperatively after formation of a loop ileostomy, followed by antegrade vancomycin flushes (500 mg in 500 mL Ringer's lactate every 8 hours) and IV metronidazole (500 mg every 8 hours) for 10 days. The biological rationale proposed for the success of this treatment protocol is based on the following: a diverting loop ileostomy interrupts the faecal stream depriving the luminal flora of nutrition; mechanical PEG lavage reduces the bacteria and toxin burden, and direct instillation of vancomycin into the colonic lumen further reduces the pathologic organism. This cohort study demonstrated a 30-day mortality of 19%, compared with 50% in the historical control group. One of the potential benefits of this regimen is that, given the reduced morbidity compared with TAC, practitioners may have been offering surgical intervention earlier in the disease process. Despite the limited sample size and methodological shortcomings of this study, this treatment regime has been adopted in some centres for select cases. A randomised controlled trial to compare this less invasive surgical approach with TAC was closed prematurely given lack of meaningful patient enrolment (clinicaltrials.gov identifier NCT01441271).
Drawing on the positive results of faecal diversion and colonic lavage proposed by Neal et al, a novel treatment strategy was instituted at London Health Sciences Centre (LHSC). This non-operative approach accomplished intestinal lavage of the colon using a nasojejunal (NJ) feeding tube to facilitate enteral delivery of PEG and vancomycin in select patients who were not considered operative candidates. Retrospective analysis of this cohort was approved by the Western University Research Ethics Board (REB File #104944). The data have not yet been published. Analysis over 24 months included 13 patients undergoing the study protocol, 9 undergoing the Pittsburgh protocol and 17 undergoing immediate colectomy. In-hospital mortality rates were 15% (2/13), 44% (4/9) and 41% (7/17) for the study protocol, Pittsburgh protocol and immediate colectomy groups, respectively. However, there are significant limitations to these data. The sample size is limited and the non-randomised, retrospective nature of the study is vulnerable to selection bias. It is possible that patients selected for the study protocol had less severe disease leading to the observed reduction in mortality. Despite the significant limitations of the data, the results are supportive and do establish equipoise regarding the optimal treatment for severe-complicated CDI. Therefore, a prospective, randomised trial is justified, especially given the significant mortality associated with fulminant CDI.
Hypothesis
In adult patients with severe or severe-complicated C. difficile infection (IDSA criteria), and additional risk factors for complications (see eligibility below) but without urgent surgical indication, intestinal lavage with PEG via NJ tube in addition to usual care will be associated with a reduced 30-day mortality rate when compared with usual care.
Trial design
The current study is a 1-year, single-centre pilot study as the first phase of a planned multicentre study. The study is a pragmatic, randomised, controlled, parallel arm, superiority trial. Patients will be randomised in a 1:1 ratio into either the intervention or control arm. The intervention will include the addition of intestinal lavage using 8 L of PEG via NJ feeding tube, to usual antibiotic treatment consisting of vancomycin (500 mg via NJ every 6 hours) and metronidazole (500 mg IV every 8 hours), both for 14 days. The comparator group will be treated with the usual antibiotic treatment alone.
Methods: participants, interventions and outcomes
Study setting
The study will be conducted over 1 year at a single tertiary care, academic health centre in London, Ontario, Canada (LHSC). Two hospital sites will be enrolling patients, with site stratification used during the randomisation process.
Screening
Study investigators are notified of all laboratory-confirmed C. difficile stool toxin assay results. All patients with a positive CDI stool test will be assessed for inclusion and exclusion criteria. Basic demographic information and screening assessments will be collected on all patients considered for enrolment.
Inclusion criteria
The study will be open to adult inpatients at LHSC with symptomatic, severe CDI with additional risk factors for fulminant or complicated infection.
Symptoms include either diarrhoea or ileus. Diarrhoea is defined as having at least three unformed bowel movements or at least 600 mL of rectal or colostomy output recorded within 24 hours on the day of or before sample collection.15 Ileus is determined clinically and radiologically by the consulting General Surgery team. Laboratory screening on stool is performed with the C. DIFF QUIK CHEK COMPLETE test (Alere Canada). The rapid cassette assay simultaneously detects glutamate dehydrogenase (GDH) antigen and toxins A and B of C. difficile in faecal specimens. The presence of GDH antigen is a screen for the presence of C. difficile. The presence of a toxigenic strain is confirmed by the presence of toxins A and B. The specimens that are GDH antigen positive but toxin A and B negative are referred for molecular testing using Loop-Mediated Isothermal Amplification (LAMP) technology illumigene (Meridian Bioscience, Canada). A positive illumigene LAMP test for toxin gene without enzyme immunoassay (EIA) toxin identification will not be considered confirmation of CDI.15
Severe CDI as defined by IDSA criteria requires either a WBC count greater than 15 000 or a serum creatinine increase of at least 50% above the premorbid level. Patients at high risk for fulminant or complicated CDI include those with either an ATLAS score11 greater than or equal to 4 (table 1), or one or more of the following objective criteria from the 2014 European Society of Clinical Microbiology and Infectious Diseases: Update of the Treatment Guidance Document for Clostridium difficile Infection10:
ATLAS score calculation11
vasopressor requirement;
mechanical ventilation requirement;
serum lactate greater than 5 mmol/L;
colonic distension (greater than 6 cm transverse colon diameter on abdominal plain film or CT);
colonic wall thickening (pancolitis) on abdominal plain film or CT, as reported by radiology.
Patient must be enrolled in the study and randomised within 72 hours of meeting study inclusion criteria.
Exclusion criteria
The Acute Care General Surgery Service will assess all patients meeting the above inclusion criteria. Patients deemed to have an immediate indication for surgery related to the diagnosis of CDI are ineligible for enrolment. In an effort to create a pragmatic study design, and to improve study compliance from all surgeons, strict indications for proceeding to surgery are not prescribed.
Other exclusion criteria include pre-existing bowel discontinuity (eg, ileostomy), contraindications to any aspect of the treatment protocol (eg, NJ feeding tube or faecal management system insertion), anticipation of patient being intolerant to treatment regimen (eg, severe ileus) or confirmed pregnancy. Additionally, patients are excluded if, despite meeting the above criteria for severe-complicated CDI, they are tolerating an enteral diet without abdominal discomfort at the time of assessment for inclusion.
Interventions
Control group
Patients randomised to the control group will receive usual antibiotic management for severe-complicated CDI as per the IDSA guidelines.6 This consists of metronidazole 500 mg IV every 8 hours and vancomycin 500 mg orally every 6 hours, both for 14 days. Patients will be nil per os (NPO) for 48 hours following randomisation. Resumption of diet after this time will be dictated by the patient's clinical picture, and will be at the direction of the consulting Acute Care General Surgery Service, and the patient's most responsible physician (MRP). All other supportive medical care will be at the discretion of the patient's MRP.
Intervention group
In addition to the usual antibiotic and supportive management described for the control group, participants randomised to the intervention arm will undergo intestinal lavage using a total of 8 L of PEG. The PEG lavage will be interrupted for 2 hours following every administration of vancomycin. Vancomycin will be administered via a NJ tube using a liquid enteral formulation.
PEG intestinal lavage requires insertion of a NJ feeding tube which follows a standardised two-step procedure. Initially, the tube is inserted to approximately 35 cm, followed by a chest radiograph to ensure that the tube is within the oesophagus and not the airway. The tube is then advanced into the proximal small bowel. Metoclopramide may be used to facilitate postpyloric positioning. If it is not possible to attain postpyloric positioning, gastric positioning is acceptable.
Stool volume assessment: Stool effluent will be monitored every 6 hours to ensure that the PEG is transiting the colon. This will require insertion of a faecal management system (eg, Flexiseal, ConvaTec, Greensboro, North Carolina, USA), or monitoring stool using a commode or continence briefs which are then weighed using a calibrated scale. Adequate colonic transit is defined as producing a stool volume of at least 50% of the volume of PEG administered over the previous 6 hours. Stool volume assessments are completed at the time of vancomycin administration.
We aim to initiate PEG lavage within 12 hours of randomisation to the study intervention arm, to allow time for tube placement and equipment set-up. Intestinal lavage with PEG is initiated and increased to a goal rate of 400 mL/hour. A total of 8 L of PEG is to be administered. In the absence of an ileus, and if the feeding tube is successfully positioned postpylorus, lavage will be initiated at 200 mL/hour. If an adequate colonic transit is demonstrated at a stool volume assessment, the PEG rate will be increased to 400 mL/hour, otherwise it will continue at 200 mL/hour.
For patients with concerns for ileus, or with prepyloric positioned feeding tubes, the lavage will be initiated at a lower rate and advanced to the goal rate more slowly. In these cases, PEG lavage will be initiated at 100 mL/hour. The stool volume will be assessed every 6 hours as above. If an adequate volume is produced, the PEG rate will be increased in 100 mL/hour increments following each adequate stool volume assessment until a goal of 400 mL/hour is achieved.
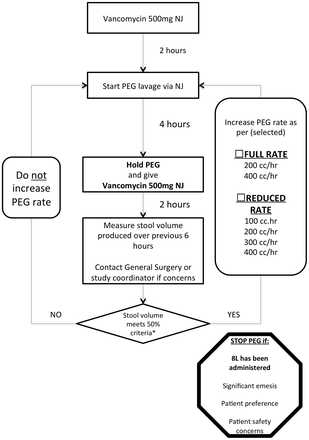
The PEG lavage and vancomycin administration schedule is further detailed as a flow sheet (see figure 1), which will appear in bedside nursing documentation. All PEG lavage will be administered with the use of the Covidien Kangaroo ePump (MedTronic, Minneapolis, Minnesota, USA).
PEG lavage and vancomycin administration schedule. NJ, nasojejunal feeding tube; PEG, polyethylene glycol. *50% criteria: rectal effluent volume is at least 50% of the volume of PEG administered over the previous 6 hours.
Duration of treatment period
The schedule of events for the trial, outlining participant screening, allocation, treatment and assessment is provided in table 2.
Schedule of events
Antibiotic treatment will be administered for a minimum total of 14 days as per established guidelines. PEG intestinal lavage should be completed within 48 hours of initiation. However, up to 72 hours to complete the lavage is considered an acceptable minor protocol violation.
The Acute Care General Surgery Service will reassess patients intermittently from the time of randomisation until completion of the intestinal lavage (or 48 hours after time of randomisation for patients assigned to the control arm). Beyond 48 hours, ongoing clinical assessment by the consulting General Surgery service will be dictated by the clinical course of the patient.
NJ feeding tubes will be removed after completion of the intestinal lavage unless required for ongoing medical care or nutritional support as dictated by the MRP caring for the patient. Faecal management systems may be removed when deemed clinically appropriate after completion of the lavage.
Following a pragmatic trial design, apart from the intervention under investigation, management will be at the discretion of the MRP and their clinical care team.
Deviations from study protocol
Definition: Protocol compliance
Full compliance with the study intervention is defined as satisfying all of the following:
Insertion of a NJ/nasogastric delivery tube with radiographic confirmation of placement.
Insertion of a faecal management system prior to initiation of PEG lavage, if deemed clinically appropriate.
Initiation of PEG lavage within 12 hours of study randomisation to the experimental arm. Antibiotics should be initiated immediately.
Completion of intestinal lavage with a total of 8 L of PEG within 48 hours from the time of lavage initiation (time zero).
Administration of dual antibiotic coverage as described.
Maintaining NPO status (except medications) for the initial 48 hours of the study protocol.
Minor protocol violations include the following:
Completion of intestinal lavage with a total of 8 L of PEG within 72 hours from the time of lavage initiation (time zero).
Failure to maintain NPO status for the initial 48 hours from protocol initiation.
Surgical intervention
Indications to escalate treatment to surgical intervention will ultimately be based on the clinical assessment by the surgical service. Absolute indications for surgery include perforation. Other indications such as toxic megacolon, worsening peritonitis or biochemical profile and inability to tolerate gastrointestinal lavage are relative indications that vary according to clinician and individual patient characteristics. To maximise surgeon uptake of the study protocol, and to fulfil the pragmatic study design, strict criteria for surgical intervention have been omitted. Surgeons’ indications for surgical intervention will be recorded.
Patient withdrawal
Study participants will be able to withdraw from the trial at any time. Unless requested in writing that all patient-specific data be withdrawn and excluded from analysis, all data collected until the time of withdrawal will be preserved for analysis. In addition, outcome data will be collected unless specifically restricted by the patient. No patient will be withdrawn from the study due to non-compliance or protocol violations and will be analysed according to intention-to-treat principles.
Discontinuation criteria
The study investigators or clinical care team may discontinue study interventions at any time for patient intolerance or complication. Such cases will be analysed following intention-to-treat principles.
Intervention interruption
Interruptions to the PEG lavage for logistic reasons such as patient transportation, imaging investigations, procedures, and so on, should be minimised. After any such interruptions, the lavage should be reinitiated at the same infusion rate as prior to cessation.
Measures to improve patient tolerance
It is anticipated that issues related to the rate of intestinal lavage will be a primary contributor to intolerance to the treatment protocol. The study protocol specifies that 8 L of PEG be administered via the NJ at a maximum of 400 mL/hour. However, if this rate is not tolerated, clinical care teams can reduce the lavage rate as necessary for tolerance and patient comfort. Protocol compliance will be assessed as defined previously. The average rate of lavage will be considered in subsequent analysis and will inform subsequent protocol modifications for future studies.
Definition: Intervention intolerance
Intolerance of the intervention treatment (PEG lavage) will be defined as either major or minor intolerance as follows:
Major intervention intolerance (any of):
Cessation of PEG lavage prior to completion of the full 8 L.
Any unplanned interruption of PEG lavage prior to completion of the full 8 L lavage, unless justified.
Justification may include logistics such as patient transport for imaging, procedure, and so on. In these cases, lavage must be reinitiated at the same infusion rate as prior to cessation.
Use of nasogastric decompression (via active suction or gravity drainage) within 72 hours of protocol initiation.
Minor intervention intolerance (any of):
Moderate to severe emesis defined as more than two episodes of emesis during the initial 72 hours of the study protocol, without satisfying any major intolerance criteria.
Any reduction in the rate of PEG lavage.
Outcomes
The outcome of interest ultimately will be 30-day mortality. Preliminary sample size calculations and anticipated accrual rate suggest that a multicentre study design is required to assess this outcome in a reasonable time frame. Therefore, this study has been designed as a 1-year feasibility pilot to assess the ability to enrol patients and successfully complete the protocol. Therefore, the primary outcome for this feasibility study will be the recruitment rate of eligible participants. Specifically, we aim to recruit an average of two patients per month, or at least 24 patients over the 1-year trial period, to support the feasibility of a larger multicentre trial.
Secondary outcomes of interest include: 30-day and 90-day all-cause mortality, surgeons’ indications for operative intervention, operative intervention rate; proportion of screened patients meeting eligibility requirements and proportion providing consent to participate, rate of compliance with study protocol, incidence of intolerance to the study protocol, complication and adverse event rate related to the study intervention. These outcomes assess protocol safety, inform modifications to the protocol for multicentre administration and provide additional data for estimates of the effect size to refine the sample size calculation for a mortality outcome.
For both the control and intervention arms, any adverse event deemed to be clinically significant by the study personnel can be recorded in a free-text format. The following specific adverse events will be monitored for and recorded explicitly:
General adverse events
Intervention for the treatment of abdominal compartment syndrome or intra-abdominal hypertension including the use of neuromuscular blockade, abdominal drain or surgical intervention within 5 days of protocol initiation
Clinical diagnosis of aspiration pneumonitis/pneumonia within 5 days of protocol initiation
New-onset seizures (theoretic risk due to electrolyte abnormalities related to PEG lavage)
NJ tube-related complications
Pneumothorax
Hollow viscous perforation
Upper gastrointestinal haemorrhage requiring endoscopic/surgical intervention or premature removal of tube
Faecal management system-related complications
Rectal haemorrhage requiring premature removal of the tube and intervention
The need for NJ feeding tube, nasogastric decompression or a faecal management system will also be recorded for the control arm, and these patients will be monitored for adverse event as described above
Strategies to maximise patient enrolment
Reducing the CDI incidence, morbidity and mortality is an institution-wide initiative at LHSC. As such, study personnel are notified directly from the microbiology laboratory of any new positive stool test for CDI. Study personnel can then approach the patient's care team and assess for study inclusion and exclusion criteria, ensuring that potential participants are not overlooked.
Sample size
Prior data indicate that the composite mortality rate among controls is 40% (0.4 as a proportion). If the true RR of mortality for experimental subjects relative to controls is 50% (based on the results of a similar intervention involving faecal diversion with a loop ileostomy and direct colonic PEG lavage14), the interventional mortality rate would be 20% (0.2 as a proportion) and 91 experimental subjects and 91 control subjects will be required to reject the null hypothesis of no difference between groups with 80% power. The type I error probability associated with this test of this null hypothesis is 0.05. However, as a feasibility study, the intent is not to demonstrate a mortality reduction with statistical significance. Rather, the intention is to show the feasibility of the protocol and to obtain an estimate of the accrual rate, which will inform the design and sample size calculation for a more definitive, and appropriately powered, trial.
Data storage and management
Data will be collected and managed using Research Electronic Data Capture (REDCap), a Health Insurance Portability and Accountability Act (HIPAA)-compliant electronic data capture tool supported and hosted by the Lawson Health Research Institute. REDCap is a secure, web-based application designed to support data capture for research studies.16 All web-based information transmission is encrypted using 128-bit encryption technology. The data are stored on a private, firewall-protected network, with servers behind the hospital firewall. Users can access the application through secure web authentication. Users are granted access based on the principle of least privilege and their access is restricted on a role-specific basis. Only members of the research team will have access to the information collected. The REDCap software package includes audit trails for tracking data manipulation, data locking and export procedures.
Data collection
All patients with a positive CDI stool test will be assessed for inclusion and exclusion criteria. Trained collectors (including study coordinators and General Surgery senior residents) will collect basic information to assess eligibility. Following enrolment and randomisations, only the trained study coordinators will record subsequent patient information and outcomes.
Randomisation
The REDCap data acquisition instruments have been programmed such that the option to randomise will only be available for patients meeting study inclusion without any exclusion criteria, and providing written consent. Randomisation then takes place within the REDCap system. Allocation tables were developed by the study statistician using a 1:1 ratio of intervention and control allocations, and block permutations of two and four patients, and stratified by hospital site. Utilising the randomisation module embedded in REDCap ensures that at the time of randomisation and patient allocation will be locked, thereby preventing any circumvention of the randomisation process.
Blinding
Patients and physicians will not be blinded. Blinding is necessary as a means to prevent postrandomisation measurement bias if the outcome is subjective. Our primary outcome (mortality) is not subjective and will occur regardless of blinding. Our secondary outcomes are somewhat subjective; however, some of the information we will be collecting is related to the decision-making process when a less invasive procedure is available. By blinding clinicians, we would be nullifying our ability to assess these important aspects of the decision-making process. Thus, bias from lack of blinding will be addressed by randomisation, allocation concealment, monitoring of cointerventions and blinding of data analysts.
Statistical analysis
We will use an intention-to-treat analysis. Data analysis will be done in a blinded fashion until all data cleaning has been performed and the statistical code for analysis has been completed.
The pilot study data will be reported in aggregate. Between-group differences will be assessed in a blinded fashion for evidence of harm, and to further refine our sample size calculation. If no significant difference is identified, and if no major protocol changes occur as a result of the pilot trial, the data will remain blinded and incorporated into the data collected for the subsequent full randomised clinical trial.
If substantive modifications to the study protocol are required following the 1-year pilot study, or significant between-group differences are identified, groups will be unblinded and analysed. In this setting, pilot study data will not be incorporated into subsequent study data.
Thirty-day and 90-day mortality will be assessed using Fisher's exact test. Secondary outcomes will be analysed using the Mann-Whitney U test (for non-normally distributed continuous variables), the Student's t-test (for normally distributed continuous variables), Fisher's exact test (for categorical variables) or the log-rank test (for time-to-event variables). Data analyses will be performed using Stata, and a two-tailed p value of <0.05 will be considered statistically significant. No correction for multiple comparisons will be used, as all of our outcomes are prespecified.
Exploratory subgroup analysis will assess the rate of both predefined and unanticipated adverse events, protocol compliance, treatment intolerance, mortality and operative intervention rate in the following subgroups:
Patients receiving care in the intensive care unit (ICU) during the initial 48 hours of the protocol, compared with those not requiring ICU care.
Patients requiring mechanical ventilator support (via endotracheal tube) before initiation of the protocol, compared with those without mechanical ventilator assistance.
Patients receiving the reduced initial rate PEG lavage for concerns of ileus, compared with those receiving the standard PEG lavage rate.
Patients with BI/NAP1/027 C. difficile strain types versus non-BI/NAP1/027 (note that this analysis will depend on the availability of further future funding for strain typing).
Data monitoring and harms
A formal Data Monitoring Committee will not be established for this feasibility study given the limited sample size and duration of the study. However, a blinded interim analysis of safety outcomes will be conducted after 6 months of patient enrolment to assess for signals of harm related to the intervention. All adverse events will be reported to our institutional REB. No efficacy outcomes will be explored at this interim analysis. If any indication of harm is identified at this interim analysis, protocol modifications, or study discontinuation, will be considered by the study investigators. Results requiring major modifications of study discontinuation will be reported to the institutional REB.
Ethics and dissemination
Protocol modifications
This study protocol has been reviewed and approved by the REB at Western University, London, Ontario, Canada.
Any modifications to the protocol which may impact on the conduct of the study, or patient safety, including changes to study design, inclusion and exclusion criteria, sample sizes, study procedures or significant administrative aspects will require a formal amendment to the protocol. Such protocol amendment would be submitted for institutional REB approval prior to implementation. Important protocol modification made following publication of the study protocol would be detailed in a final manuscript at the time of publishing study results.
Consent process
Eligible patients identified for study participation will be provided with a letter of information (LOI) outlining the study rationale, interventions and potential risks and benefits. In the event that potential participants are unable to participate in the consent process (eg, intubated or incapacitated), a substitute decision maker may participate in the consent process on behalf of the patient. Our institutional REB has approved the LOI. Consent to participate may be sought by the study investigators, coordinators or representative General Surgery residents. The study LOI and consent form also include consent for collection of stool specimens for subsequent ancillary studies assessing vancomycin concentrations in the stool, C. difficile colony counts and C. difficile strain typing.
Personal health information
Data fields pertaining to personal health information have been preidentified in REDCap. Limited user access rights within REDCap restrict access to these fields to data entry only, for the majority of users. The principle investigator and coinvestigator retain access to the entire data set. Data export for statistical analysis is limited to non-personal health identifiers data fields only.
Data collected for potential study participants only include that necessary to assess for study inclusion and exclusion criteria. Additional data such as organ injury severity scores, comorbidities and outcomes will only be collected for those patients providing written informed consent and undergoing randomisation.
Dissemination of results
Following completion of the study and final analysis, data will be presented to the medical community through publication in a peer-reviewed scientific journal, as well as national and international surgical and medical scientific conferences. Results will also be presented to the Canadian Critical Care Trials Group in anticipation of a multicentre trial.
Authorship for the final manuscripts submitted for publication will require substantive contribution to the work and will follow the recommendations of the International Journal of Medical Journal Editors. We do not anticipate the use of a professional writer during manuscript development.
Discussion
The major operational issue anticipated with the protocol is efficient patient identification and protocol administration, as these will impact the local study as well as the proposed multicentre trial. C. difficile can affect inpatients throughout the hospital under the care of any service. Local institutional guidelines suggest that the Acute Care General Surgery Service be involved in the care of all inpatients with severe CDI. Therefore, to maximise enrolment, study investigators are notified directly from the microbiology laboratory of all positive C. difficile stool toxin tests. Furthermore, overwhelming support for the study from the General Surgery service at LHSC was confirmed when assessed in an online survey. Fifteen of 22 consultant surgeons at LHSC responded to the survey and of those respondents, 100% indicated a willingness to enrol patients and participate in the study.
In order to demonstrate a mortality reduction, we anticipate requiring at least 91 patients in each study arm (see Sample Size calculation). If enrolment of two patients per month is demonstrated in the present study, a multicentre trial utilising five sites would require 1.5 years to be completed. Furthermore, to be successful at other sites, significant support from General Surgery services at those centres would be required. Therefore, the protocol has been designed to limit the workload and duration of involvement required of those services. However, we do anticipate that a ‘run-in’ period of several months, or an additional pilot multicentre study involving a limited number of distributed sites, may be necessary prior to initiation of the planned definitive multicentre study.
Trial status
Recruitment for this trial opened in August 2016 and is planned to close August 2017. At the time of manuscript submission, the trial was in the recruitment phase.
References
Footnotes
Contributors GM conceived and designed the study protocol, submitted and obtained grant funding to support the study and drafted the manuscript. PMJ made substantial contributions to designing the study protocol, developed the study database within REDCap and planned the statistical analysis. BK developed the experimental treatment concept, made substantial contributions to designing the study protocol and helped draft the manuscript. VD assisted with the manuscript development and editing. TM made substantial contributions to designing the study protocol, submitted and obtained grant funding to support the study, helped draft the manuscript and is the Principle Investigator for the study. GM and TM had the final responsibility for the decision to submit for publication.
Funding Lawson Health Research Institute, Schulich School of Medicine & Dentistry, London Health Sciences Centre.
Competing interests None declared.
Ethics approval Western University (London, Ontario, Canada) Institutional Review Board. REB file #104944.
Provenance and peer review Not commissioned; externally peer reviewed.