Article Text
Abstract
Background/objectives Evidence suggests that aerobic exercise may slow the progression of subcortical ischaemic vascular cognitive impairment (SIVCI) by modifying cardiovascular risk factors. Yet the economic consequences relating to aerobic training (AT) remain unknown. Therefore, our primary objective was to estimate the incremental cost per quality-adjusted life years (QALYs) gained of a thrice weekly AT intervention compared with usual care.
Design Cost–utility analysis alongside a randomised trial.
Setting Vancouver, British Columbia, Canada.
Participants 70 adults (mean age of 74 years, 51% women) who meet the diagnostic criteria for mild SIVCI.
Intervention A 6-month, thrice weekly, progressive aerobic exercise training programme compared with usual care (CON; comparator) with a follow-up assessment 6 months after formal cessation of aerobic exercise training.
Measurements Healthcare resource usage was estimated over the 6-month intervention and 6-month follow-up period. Health status (using the EQ-5D-3L) at baseline and trial completion and 6-month follow-up was used to calculate QALYs. The incremental cost–utility ratio (cost per QALY gained) was calculated.
Results QALYs were both modestly greater, indicating a health gain. Total healthcare costs (ie, 1791±1369 {2015 $CAD} at 6 months) were greater, indicating a greater cost for the thrice weekly AT group compared with CON. From the Canadian healthcare system perspective, the incremental cost–utility ratios for thrice weekly AT were cost-effective compared with CON, when using a willingness to pay threshold of $CAD 20 000 per QALY gained or higher.
Conclusions AT represents an attractive and potentially cost-effective strategy for older adults with mild SIVCI.
Trial registration number NCT01027858.
- mild cognitive impairment
- cost-utility analysis
- economic evaluation
- older adults
- exercise
- aerobic training
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
- mild cognitive impairment
- cost-utility analysis
- economic evaluation
- older adults
- exercise
- aerobic training
Strengths and limitations of this study
Our study is one of the first to investigate together the economic and health consequences relating to aerobic training.
Very few randomised controlled trials with concurrent economic evaluations of exercise have been conducted in populations at risk for dementia such as those with vascular cognitive impairment.
There was wide variability in the cost estimates and outliers due to a smaller sample size.
Introduction
Cerebrovascular disease is the second most common aetiology contributing to dementia in older adults1–4 and may be the most underdiagnosed and yet most treatable form of cognitive dysfunction in older adults.5 Vascular cognitive impairment (VCI)—defined as the loss of cognitive function due to vascular burden in the brain—is a prevalent condition that places a growing burden on the healthcare system.6 Cerebral small vessel disease plays a critical role in covert ischaemia and the development of subcortical ischaemic vascular cognitive impairment (SIVCI),7 the most common form of VCI_.8 SIVCI is defined by the presence of white matter lesions (WMLs) and lacunar infarcts and has the clinical consequence of increased dementia risk.8 ,9 Research has demonstrated that one-third of all dementias are attributable to VCI.10–12 More specifically, the proportion of vascular dementia attributable to small-vessel disease ranges from 36 to 67%.13 ,14 The worldwide economic burden of dementia is increasing at an unprecedented rate. In 2015, a 35% increase led to a worldwide annual estimate of 818 billion US dollars. The worldwide costs of dementia are expected to exceed 1 trillion US dollars by 2018.15 Notably, vascular dementia has among the highest annual direct costs and highest hospitalisation-related costs compared with other dementias such as Alzheimer’s disease.16 The average annual cost per patient with VCI was $33 740,6 compared with a variable range of $1500–$91 000 for Alzheimer's disease. The costs per VCI admission were ∼$9545 with the average number of admissions increasing through the progression of the disease.6
Epidemiological data suggest that modification of vascular risk factors may be beneficial in slowing the progression of VCI.17–20 Hence, aerobic-based exercise training is one promising approach to delay the progression of VCI by reducing key vascular risk factors associated with metabolic syndrome. What remains unknown is whether aerobic-based exercise training as an intervention strategy compared with ‘usual care’ for individuals with mild SIVCI is a cost-effective strategy. Until now, the simultaneous impact of healthcare costs and consequences remains unknown. It is an essential next step to provide an estimate of the costs and consequences (ie, health gains or losses) related to the aerobic training (AT) intervention, given that this type of intervention could be delivered at a population level and thus have an immense impact.
Therefore, we conducted a concurrent economic evaluation with individual level data on cost and effectiveness outcomes collected during a proof-of-concept single-blinded randomised controlled trial—the Promotion Of the Mind Through Exercise (PROMoTE) trial.21 Our primary objective was to determine the incremental cost–utility ratio (incremental cost per incremental quality-adjusted life year (QALY) gained) of thrice weekly AT compared with usual care among individuals with mild SIVCI.
Methods
Overview of economic evaluation
This cost–utility analysis was conducted concurrently with a 6-month proof-of-concept single-blinded randomised controlled trial with a 6-month follow-up study (ie, 6 months postintervention).21 ,22 The details of the PROMoTE trial were previously reported.21 ,22 Measurements were made at three times: baseline, end of the intervention period (6 months postrandomisation) and 6-month postintervention (ie, 12 months postrandomisation). Of 440 individuals screened for eligibility, 70 were deemed eligible for this economic evaluation. This economic evaluation used a Canadian healthcare system perspective, and a 6-month (ie, trial completion) and a 12-month (ie, 6-month postintervention) time horizon for the primary economic evaluation assessing the efficiency of the thrice weekly progressive AT and usual care plus education compared with the usual care plus education (CON; comparator) group. Participants in the CON group received usual care as well as monthly educational materials about VCI and healthy diet. However, no specific information regarding physical activity was provided. Briefly, usual care included whatever healthcare services a patient with mild SIVCI would usually receive in their clinical care. The main outcome for the cost–utility analysis was the incremental cost per QALY gained. We obtained approval for this study from the University of British Columbia Clinical Ethics Review Board (H13-00715).
We previously described study design, participant recruitment, randomisation, demographics, methods and results of the PROMoTE trial.21 We recruited participants from the University of British Columbia Hospital Clinic for AD and related disorders, the Vancouver General Hospital Stroke Prevention Clinic and specialised geriatric clinics in Metro Vancouver, BC. Recruitment occurred between December 2009 and April 2014 with randomisation occurring on an ongoing basis. The assessors were blinded to the participants' group allocation. The primary outcome measures for the PROMoTE study were the Alzheimer's Disease Assessment Scale Cognitive Subscale (ADAS-Cog23), the Executive Interview (EXIT-2524) and the Alzheimer's Disease Co-operative Study—Activities of Daily Living (ADCS-ADL25). Secondary outcome measures included executive functions, cardiovascular capacity, physical activity level, physiological markers and health-related quality of life. We included 70 community dwelling older adults who were diagnosed with SIVCI,26 which requires the presence of cognitive syndrome21 and small vessel ischaemic disease.21 Other inclusion criteria included: (1) Montreal Cognitive Assessment (MoCA)27 score <26 at screening; (2) Mini-Mental State Examination (MMSE)28 score of ≥20 at screening; (3) community-dwelling; (4) live in Metro Vancouver; (5) had a caregiver, family member or friend who interacted with him/her on a weekly basis; (6) sufficient ability to read, write and speak English; (7) acceptable visual and auditory acuity to complete psychometric tests; (8) stable on a fixed dose of cognitive medications that is not expected to change during the 6-month intervention period; (9) provided a personally signed and dated informed consent document indicating that the individual (or a legally acceptable representative) has been informed of all pertinent aspects of the trial; (10) able to walk independently and (11) in sufficient health to participate in the study’s aerobic-based exercise training programme.
Costs
We tracked healthcare resource usage prospectively. Our primary method used cost diaries where participants were asked to fill out a monthly diary detailing any health resource usage. We also telephoned participants every 3 months using a health resource usage questionnaire. For individuals who did not fill out their calendars, the health resource usage questionnaire was the primary mode of healthcare resource usage data collection. For participants who missed the 3-month follow-up telephone call and who did not return their calendar, they were asked to recall their healthcare resource usage over the 6-month intervention period. We also collected healthcare resource usage for the 6-month follow-up period postintervention. We analyse these end points separately (ie, trial completion at 6 months and follow-up completion at 1 year). The healthcare resource usage questionnaire included the following categories: any visits to healthcare professionals (including general practitioners, specialists, physiotherapists, etc); all visits, admissions or procedures carried out in a hospital and laboratory and diagnostic tests. We calculated the costs of delivering the thrice weekly AT intervention and the CON group. Our base case analysis considered the costs of all healthcare resource use. Research protocol-driven costs were excluded from our analysis.
A unit cost was assigned for each component of healthcare resource usage. Costs for admission to hospital were based on the fully allocated cost model of a tertiary care hospital, Vancouver General Hospital. We based costs on fee for service rates from the British Columbia Medical Services Plan 2013 price list for all healthcare professional-related costs. Unit costs for specialised services (ie, physiotherapy, chiropractic or naturopathic medicine) were taken from the BC Association website for each specialty. We inflated costs to 2015 Canadian dollars using the consumer price index reported by Statistics Canada. Given that our analytic time horizon was ≤12 months, discounting was not applied.
Effectiveness outcome
Briefly, we assessed health status using the EQ-5D-3L.29 The EQ-5D-3L is a short five-item multiple choice questionnaire that measures an individual's health-related quality of life (HRQoL) and health status according to the following five domains: mobility, self-care, usual activities, pain and anxiety/depression.29 Each domain has three possible options: no problems, some problems or severe problems. The EQ-5D-3L health state utility values (HSUVs) at each time point are bounded from −0.54 to 1.00 where a score of <0 is indicative of a health state worse than death. The HSUVs represent values that individuals within society assign––these are Canadian societal values for given health states.30
We administered the EQ-5D-3L at baseline, trial completion and at the 6-month follow-up period to patients and a patient proxy (ie, see below under ‘Caregiver’). From these data points, we calculated the total QALYs lost or gained at 6 (trial completion) and 12 (follow-up completion) months for the two experimental groups. We used multiple linear regression to calculate the incremental QALYs based on patient and proxy ratings for each participant adjusted for the baseline utility score. Baseline utility scores are often imbalanced between treatment arms. Given that a patient's utility score at baseline is most often highly correlated with that individual's QALYs over the study period, failure to control for this imbalance can lead to a misleading ICER. As such, we followed the recommendations of Manca and Palmer31 using multiple linear regression to control for imbalances in baseline utility scores between the two treatment groups. All statistical analyses were carried out using STATA V.10.0.
Caregiver (proxy)
The caregivers had to be able to read, write and speak English in which the questionnaires were provided with acceptable visual and auditory acuity. Caregivers completed the EQ-5D-3L from their own perspective of the participant (ie, proxy's own perspective).
Adverse events and mortality
Participants were advised to report any adverse effects due to the intervention. Our safety monitoring committee reviewed all adverse events on a monthly basis.
Handling missing data
In the PROMoTE study, 17% of participants had incomplete 6-month health resource usage data and 7% had incomplete 6-month EQ-5D-3L data at trial completion including dropouts. For the 6-month follow-up period, 19% of participants had incomplete 6-month follow-up health resource usage data and 19% had incomplete 6-month follow-up EQ-5D-3L data. The reasons for missing data included: drop out, participant burden and administration error. We calculated the cost and effectiveness estimates for available cases (dropping observations with missing values), complete case sets and an imputed data set.
We examined the pattern of missing data using the STATA code: ‘mvpatterns’. Missing data appeared to be missing at random, and therefore, we imputed missing data using Bayesian analyses following recommendations,31–34 ,35 in which all baseline study variables (including treatment assignment) were used to create 40 imputed data sets; parameter estimates and SEs were pooled across the 40 data sets. For multiple imputation, we used the ‘mi imput mvn’ procedure in STATA. The imputed data are reported as our base case analysis. We report the results using deletion of missing data as our sensitivity analysis (ie, complete case analysis).
Cost–utility analysis
We calculated the incremental cost–utility ratio for thrice weekly AT compared with the CON group twice using the patient-rated EQ-5D-3L and the caregiver proxy-rated EQ-5D-3L. Briefly, the incremental cost–utility ratio provides an index of the cost per QALY gained at intervention completion (ie, 6 months) and cost per QALY gained at 6-month postintervention (ie, 12 months). The incremental cost–utility ratio is the ratio between the difference in total mean costs between the AT and the CON groups and the difference in the mean QALY gained between the AT and the CON groups. 1
1
Nested imputation and nonparametric bootstrapping were used to model uncertainty around the estimates for costs and effectiveness. For each of the 40 cycles, we imputed missing values and bootstrapped the complete data set. For each cycle of imputation and bootstrapping, we calculated the total healthcare resource use cost and QALYs according to group allocation. The results of each cycle of imputation for participants were averaged in each of the two participant groups. The contribution of each cost item in relation to the total healthcare resource use was estimated for each group. Plots of the cost-effectiveness plane and cost-effectiveness acceptability curves were generated based on 5000 iterations of nested imputation/bootstrapping using Fiellers' method to generate 95% confidence ellipses for the joint distribution of cost and effectiveness outcomes.36
The differences in mean costs and health outcomes in each group were expressed by reporting the incremental cost per QALY (ie, the incremental cost–utility ratio). The observed health benefit (ie, QALY) difference was close to zero; therefore, we used 5000 bootstrapped replications of mean cost and QALY differences.37 We used these values to generate cost–utility acceptability curves to estimate the probability that thrice weekly AT is considered cost-effective compared with CON over a select range of willingness to pay values.38
Sensitivity analysis
For our sensitivity analysis, we restricted our data to a complete case analysis, thus including only participants for whom we had complete cost and effectiveness data. We applied multiple imputation, bootstrapped CI estimation, adjustment for imbalances in baseline utility and bootstrapped estimates of the incremental cost–utility ratios.
Results
Baseline characteristics and exercise compliance
Seventy-one eligible participants were randomised to AT or CON. One participant was deemed ineligible due to the presence of mixed dementia detected after randomisation and was excluded from all analyses. As such, our analytic sample consisted of 70 participants. Table 1 provides the baseline descriptive characteristics separated by the study group. Average class attendance was 68% for the AT group.
Baseline characteristics of participants
Healthcare use and costs
Complete healthcare resource usage data were provided by 58 (83%) participants at 6 months and 57 (81%) participants at 12 months. Response rates for healthcare usage data were comparable across the two participant groups. Unit costs for healthcare cost items are provided in table 2. In summary (table 2), the mean (SD) costs (2015 $CAD) for healthcare professional visits, admissions to hospital and laboratory tests/investigations at 6 months were as follows: 940 (1194), 187 (325) and 113 (128). The mean (SD) costs (2015 $CAD) for healthcare professional visits, admissions to hospital and laboratory tests/investigations at 6 months were as follows: 682 (465), 554 (1648) and 108 (132). The mean total healthcare resource usage costs for the control group (2015 $CAD) at 6 and 12 months were 1434 (1674) and 2964 (2947). The mean total healthcare resource usage costs for the AT group (2015 $CAD) at 6 and 12 months were as follows: 1434 (1674) and 2964 (2947).
Unit costs for each component of resource usage
Health outcomes
Complete data for the EQ-5D-3L at baseline were provided by 69 (99%) patients and 63 (90%) caregivers. Complete data for the EQ-5D-3L at 6 months were provided by 65 (93%) patients and 54 (77%) caregivers. Complete data for the EQ-5D-3L at 12 months were provided by 57 (81%) patients and 49 (70%) caregivers. The response rates of patients or caregivers for dropouts were comparable between treatment groups. The mean EQ-5D-3L at 6 and 12 months and adjusted incremental QALYs for patients and caregivers are provided in table 3.
Results of imputed case analysis
Adjusting QALYs for imbalances in baseline utility
Imputed case analysis
After controlling for imbalances in baseline utility, the mean (SD) incremental QALY after 6 months calculated using the EQ-5D-3L was 0.82 (0.06) as rated by patients and 0.83 (0.06) as rated by the caregivers’ perspective for the patients in the AT group and 0.78 (0.09) as rated by patients and 0.79 (0.12) as rated by the caregivers’ perspective for the patients in the CON group (table 3). After controlling for imbalances in baseline utility, the mean (SD) incremental QALY after 12 months calculated using the EQ-5D-3L was 0.82 (0.06) as rated by patients and 0.83 (0.05) as rated by the caregivers’ perspective for the patients in the AT group and 0.78 (0.08) as rated by patients and 0.79 (0.10) as rated by the caregivers’ perspective for the patients in the CON group (table 3).
Complete cases analysis
After controlling for imbalances in baseline utility, the mean (SD) incremental QALY after 6 months calculated using the EQ-5D-3L was 0.82 (0.06) as rated by patients and 0.83 (0.05) as rated by the caregivers’ perspective for the patients in the AT group and 0.78 (0.09) as rated by patients and 0.78 (0.12) as rated by the caregivers’ perspective for the patients in the CON group (table 3). After controlling for imbalances in baseline utility, the mean (SD) incremental QALY after 12 months calculated using the EQ-5D-3L was 0.82 (0.03) as rated by patients and 0.82 (0.01) as rated by the caregivers’ perspective for the patients in the AT group and 0.78 (0.05) as rated by patients and 0.78 (0.03) as rated by the caregivers’ perspective for the patients in the CON group.
Cost–utility analysis
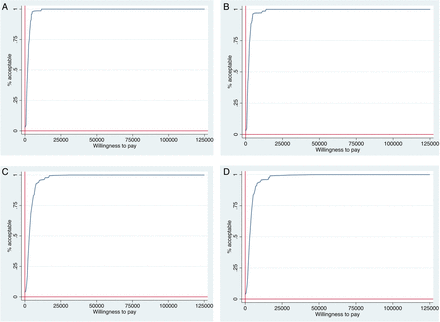
From the Canadian healthcare system perspective, the incremental cost–utility ratios for thrice weekly AT were cost-effective compared with the comparator group, when using a willingness to pay threshold of $CAD 20 000 per QALY gained or higher. Specifically, on the point estimates from our base case analysis, we found that AT is more effective and also more costly than the CON alternative. Figure 1A (using the patient’s own ratings of their health status) demonstrates that for three times weekly AT at 6 months (ie, intervention completion) compared with CON, most of the bootstrapped cycles (>80% of the 4000 cycles) were represented in the northeast quadrant. Figure 1B (using the caregiver’s ratings of the patient’s health status) demonstrates that for three times weekly AT at 6 months (ie, intervention completion) compared with CON, most of the bootstrapped cycles (>80% of the 4000 cycles) were represented in the northeast quadrant. Figure 1C, D (using the patient’s own ratings and caregiver's ratings of their health status, respectively) demonstrates that for three times weekly AT at 12 months (ie, intervention completion) compared with CON, most of the 4000 bootstrapped cycles were represented in the northeast quadrant. Figure 2A–D reports the cost-effectiveness acceptability curves highlighting the probability of the AT being cost-effective over different willingness to pay values.
(A) Cost-effective plane (time horizon—6 months) depicting the 95% confidence ellipses of incremental cost and effectiveness (patient-rated health status) for comparison between thrice weekly aerobic training and usual care (control, comparator). (B) Cost-effective plane (time horizon—6 months) depicting the 95% confidence ellipses of incremental cost and effectiveness (caregiver (patient-proxy) rated health status) for comparison between thrice weekly aerobic training and usual care (control, comparator). (C) Cost-effective plane (time horizon—12 months) depicting the 95% confidence ellipses of incremental cost and effectiveness (patient-rated health status) for comparison between thrice weekly aerobic training and usual care (control, comparator). (D) Cost-effective plane (time horizon—12 months) depicting the 95% confidence ellipses of incremental cost and effectiveness (caregiver (patient-proxy) rated health status) for comparison between thrice weekly aerobic training and usual care (control, comparator).
(A) Cost-effectiveness acceptability curve showing the probability that thrice aerobic training intervention is cost-effective compared to usual care over a range of values for the maximum acceptable ceiling ratio (λ—willingness to pay) in the PROMoTE trial (6-month time horizon, patient-rated health status). (B) Cost-effectiveness acceptability curve showing the probability that thrice aerobic training intervention is cost-effective compared to usual care over a range of values for the maximum acceptable ceiling ratio (λ—willingness to pay) in the PROMoTE trial (6-month time horizon, caregiver (patient-proxy)-rated health status). (C) Cost-effectiveness acceptability curve showing the probability that thrice aerobic training intervention is cost-effective compared to usual care over a range of values for the maximum acceptable ceiling ratio (λ—willingness to pay) in the PROMoTE trial (12-month time horizon, patient-rated health status). (D) Cost-effectiveness acceptability curve showing the probability that thrice aerobic training intervention is cost-effective compared to usual care over a range of values for the maximum acceptable ceiling ratio (λ—willingness to pay) in the PROMoTE trial (12-month time horizon, caregiver (patient-proxy) rated health status).
Sensitivity analysis
Our complete case analysis demonstrated the same trend with regard to a significant improvement in QALYs and an overall increase in health resource usage costs for the AT group.
Discussion
Among a population of individuals at high risk for future cognitive decline, this study demonstrated, using a Canadian healthcare system perspective, that the incremental cost per QALY gained by participating in thrice weekly AT was more effective and more costly than the usual care plus education group. We observed a trend towards improvement in the adjusted incremental QALYs determined from the EQ-5D-3L (by patients and proxies) for the AT group compared with the usual care plus education group at trial completion and 6-month follow-up. Importantly, AT is an alternative to resistance training and is accessible to older adults with mild SIVCI. Further, the delivery of a walking programme on an individual basis requires a low financial investment (ie, the cost of walking poles) by an individual. As such, the results of this economic evaluation represent a substantive contribution to the evidence base on how to efficiently minimise cognitive decline among those with mild SIVCI.
The findings of this study build on previous research demonstrated that AT has significant and beneficial effects on overall health-related quality of life and quality of life more broadly.39 The overall incremental cost–utility ratios were not significantly different regardless of whether QALYs were ascertained from patient-reported or proxy-reported health status using the EQ-5D-3L suggesting that for this population, use of patient or proxy ratings should not alter healthcare decision-making. The cost-effectiveness acceptability curves confirm that AT is the preferred treatment option for a wide range of plausible willingness to pay thresholds.
From both our sensitivity analyses, we found that all analyses supported the conclusions that AT resulted in clinically important gains in QALYs. However, our imputed case analysis demonstrated that the intervention was not cost-saving, while the complete case analysis demonstrated that the intervention was cost-saving. One potential explanation for this was that the complete case analysis may better reflect the per protocol findings (ie, those that had greater adherence to the trial).
The time horizon of our study was limited to the duration of the intervention (ie, 6 months) and the follow-up period (ie, 12 months). The number of randomised controlled trials of exercise conducted in populations at high risk for dementia is accumulating.40 One study demonstrated that resistance training post a 6-month (frequency of two to three times weekly) intervention significantly improved global cognitive function while maintaining executive and global benefits for at least 18 months postintervention.41 ,42 However, the number of randomised controlled trials of exercise among individuals diagnosed with SIVCI remains low.22 Given that cardiovascular risk factors play a primary role in the onset and progression of VCI, examining the cost-effectiveness of AT is a logical starting place. In adults with mild VCI, 6 months of thrice weekly progressive AT improved cognitive function, relative to CON.22 Previous research that AT in older adults has longer term health benefits that we hypothesise would be applicable to adults with mild SIVCI benefit of the intervention may be ideally captured by a longer time horizon.43 Further, the sample size of our study was small. As such, there was wide variability in the cost estimates and outliers (ie, ±3 SDs from the mean) had a stronger impact than would be expected in a larger sample. However, we did not have any reason to remove any health resource usage outliers in our intention to treat analysis. The health resource usage questionnaire may be subject to recall bias, thus causing potential underestimation of costs. To minimise recall bias, participants were provided with a monthly diary to track and report their healthcare resource usage. Given that cost underestimation may have occurred in both groups, we do not estimate any impact on the incremental cost–utility ratio given that this was a randomised controlled trial.
A key strength of our study is that it deals with a largely understudied yet important population. Importantly, this population actually may represent an ideal target population for intervention given that individuals have not yet crossed the dementia threshold. Hence, it is important to gain a better understanding of the effectiveness and efficiency of targeted interventions. Given that even mildly impaired cognition may impede an individual's ability to self-assess their HRQoL we also used a patient-proxy (ie, caregiver) assessment of the patient’s health status.44 ,45 In this study, we found that the use of the patient or the proxy did not significantly alter our findings. In all instances, we observed a significant increase in QALYs at 6 and 12 months regardless of the rater. This is a useful observation because it suggests that among individuals with VCI, the rater should not result in changes in healthcare decision-making. Finally, a highly relevant strength of this study is that the intervention is widely accessible and relatively easy to implement for any community dwelling older adult who is able to walk. The low cost required by an individual to start walking is also appealing from an implementation perspective.
Our proof-of-concept findings suggest that this exercise (ie, AT) therapy delivered over a span of 6 months holds promise for improving cognitive function and health-related quality of life in older adults with mild VCI. While our findings suggest that this intervention is not cost-saving, it appears to be cost-effective depending on a decision maker’s willingness to pay.
Acknowledgments
The authors thank the PROMoTE study participants.
References
Footnotes
Contributors TL-A and JCD had full access to all the data in the study, take responsibility for the integrity of the data and the accuracy of the data analysis and involved in study concept and design. TL-A, JCD, SB and JRB contributed to acquisition, analysis or interpretation of data. JCD, TL-A and SB drafted the manuscript. JCD, G-YRH, SB, JRB, JJE, MM, WC, BC, CJ, PL and TL-A contributed to critical revision of the manuscript for important intellectual content. JCD and JRB involved in statistical analysis. TL-A, CJ, G-YRH, JJE, PL and JCD obtained funding. WC, MM and BC provided administrative, technical, or material support. TL-A, JCD, MM and WC contributed to study supervision.
Funding This study is jointly funded by the Canadian Stroke Network and the Heart and Stroke Foundation of Canada (grant number: PG-09-0443). TL-A is a Canada Research Chair in Physical Activity, Mobility, and Cognitive Neuroscience, a Michael Smith Foundation for Health Research (MSFHR) Scholar, a Canadian Institutes of Health Research (CIHR) New Investigator and a Heart and Stroke Foundation of Canada's Henry JM Barnett's Scholarship recipient. These funding agencies did not play a role in study design.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Unpublished data are available on request. Please email Dr Teresa Liu-Ambrose: teresa.ambrose@ubc.ca.