Article Text
Abstract
Introduction Pulmonary vein isolation (PVI) is an effective therapy for paroxysmal atrial fibrillation (AF), but it has limitations. The two most significant recent advances have centred on the integration of real-time quantitative assessment of catheter contact force into focal radio frequency (RF) ablation catheters and the development of dedicated ablation tools capable of achieving PVI with a single ablation lesion (Arctic Front cryoballoon, Medtronic, Minneapolis, MN, USA). Although each of these holds promise for improving the clinical success of catheter ablation of AF, there has not been a rigorous comparison of these advanced ablation technologies. Moreover, the optimal duration of cryoablation (freezing time) has not been determined.
Methods and analysis Patients undergoing an initial PVI procedure for paroxysmal AF will be recruited. Patients will be randomised 1:1:1 between contact-force irrigated RF ablation, short duration cryoballoon ablation (2 min applications) and standard duration cryoballoon ablation (4 min applications). The primary outcome is time to first documented AF recurrence on implantable loop recorder. With a sample size of 111 per group and a two-sided 0.025 significance level (to account for the two main comparisons), the study will have 80% power (using a log-rank test) to detect a difference of 20% between contact force RF catheter ablation and either of the two cryoballoon ablation groups. Factoring in a 4% loss to follow-up, 116 patients per group should be randomised and followed for a year (total study population of 348).
Ethics and dissemination The study was approved by the University of British Columbia Office of Research (Services) Ethics Clinical Research Ethics Board. Results of the study will be submitted for publication in a peer-reviewed journal.
Trial registration number NCT01913522; Pre-results
- atrial Fibrillation
- ablation
- cryoablation
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Strengths and limitations of this study
The CIRCA-DOSE is the first large multicentre randomised trial exclusively evaluating modern ablation technologies (contact-force-guided radio frequency ablation compared with second-generation cryoballoon ablation).
A major strength of the trial is the rigour to which arrhythmia outcomes will be evaluated. In addition to continuous arrhythmia monitoring, all arrhythmia events will be independently adjudicated by a committee blinded to treatment allocation, and thus it represents one of the most robust AF ablation outcome trials performed to date.
The trial is designed to evaluate outcomes beyond dichotomous arrhythmia recurrence, including AF burden (which is impossible to quantify with intermittent rhythm monitoring techniques) and quality-of-life metrics.
The inclusion criteria were designed to mimic the patients seen in clinical practice (including the inclusion of patients with persistent AF) in order to ensure that the trial is externally valid and generalisable.
Although powered for arrhythmia recurrence outcomes, the relatively limited sample size will limit future subanalyses.
Introduction
Atrial fibrillation (AF) is a common chronic progressive disease characterised by exacerbations and remissions. Over the past 10–15 years, multiple large-scale observational studies and randomised controlled trials have demonstrated that catheter ablation is superior to antiarrhythmic drug (AAD) therapy in maintaining sinus rhythm.1–10 In addition, catheter ablation has been shown to be superior to AADs for the improvement of symptoms, exercise capacity and quality of life.4 11–13 Unfortunately, the results of ablation are limited by arrhythmia recurrence, which is most often due to a failure to effectuate a durable contiguous circumferential transmural myocardial lesion around the pulmonary vein (PV) ostia.1 3–10 14 15 In response, considerable effort has been directed towards developing technologies to achieve safer and more durable PV isolation (PVI). The two most significant advances in the last few years have centred on the integration of real-time quantitative assessment of catheter contact force into focal radio frequency (RF) ablation catheters, and the development of dedicated catheters capable of achieving PVI with a single ablation lesion, the most mature of which is the Arctic Front cryoballoon (Medtronic, Minneapolis, MN, USA).
Although each of these advances holds promise for improving the clinical success of catheter ablation of AF, there has not been a rigorous comparison of the contact-force-assisted RF ablation versus the second-generation cryoballoon. The CIRCA-DOSE trial has been designed to evaluate these two questions. The CIRCA-DOSE study is a multicentre randomised trial designed to rigorously evaluate the effectiveness of contact-force-assisted RF PVI versus PVI performed with the second-generation cryoballoon, as well as evaluate the optimal cryoablation duration.16
Contact force ablation
Ablation electrode-tissue contact is an important determinant of lesion size, and ultimately durability of conduction block. Conventionally, this has been assessed by the operator using a combination of fluoroscopic imaging of the catheter tip motion, tactile feedback and local electrogram attenuation, as well as impedance reductions during energy delivery. Although widely used, the accuracy of these surrogate measures is poor. Contact force sensing is a recent innovation that allows for real-time estimation of the contact force between the tip of the catheter and the target myocardium, thus providing the operator with an accurate quantitative assessment of tissue contact.
Recent data suggest that incorporating real-time contact force assessment results in a reduction in procedure time, ablation time and total energy delivery, with a comparable safety profile to that observed with standard irrigated RF.17 18 However, the two largest multicentre trials evaluating this technology demonstrated a 1-year success of 68% (TactiCath, TOCCASTAR) and 74% (SmartTouch, SMART-AF).19 20 In the case of the former, the success was no different from that observed with standard non-contact force RF ablation. Interestingly, post hoc analyses of these studies suggested that the outcomes were improved when the procedure was performed with adequate contact force parameters (84% 1-year freedom from AF in the 47% of patients in whom ablation was in the target range ≥80% of the time in SMART-AF, and 76% 1-year freedom from AF in the 57% of patients in whom ≥90% of the lesions were >10 g in TOCCASTAR). No differences in the incidence of complications have been reported between patients undergoing ablation with contact force versus non-contact force sensing RF ablation catheters.19 21 22
Cryoballoon ablation
Recent studies have examined short-term and long-term success with the second-generation cryoballoon. Studies of planned remapping procedures have demonstrated that the durability of PVI at 3 months post-index ablation procedure was improved at 91% with the second-generation cryoballoon, compared with 67% of PVs with standard (non-contact force) RF and 88% of PVs with the first-generation cryoballoon.18 23–27 Clinically, this has translated into a 1-year freedom from recurrent AF of 82% with the second-generation cryoballoon (11 studies; 1725 patients), which was significantly improved compared with the first-generation cryoballoon in a separate comparative meta-analysis (OR of arrhythmia recurrence 0.34 (0.26–0.45) when compared with first-generation cryoballoon; 10 studies, 2310 patients).28 From a safety standpoint, there were significantly more phrenic nerve palsies (transient and persistent) observed with the second-generation cryoballoon.
Contact force ablation versus cryoballoon ablation
There is limited data directly comparing contact-force-guided RF ablation to cryoballoon ablation. Since the inception of the CIRCA-DOSE study, three observational studies have reported comparable safety and efficacy between contact-force-guided RF ablation and cryoballoon ablation for paroxysmal (two studies) and persistent (one study) AF. Specifically, Jourda et al reported a single-centre experience with 150 consecutive patients undergoing PVI for paroxysmal AF with the second-generation cryoballoon (75 patients) and contact-force-guided irrigated RF ablation (SmartTouch, 75 patients).29 In this non-randomised study, the 1 -year freedom from recurrent AF (as detected by Holter monitoring at 1, 3, 6, 9 and 12 months) was 85% in the cryoballoon group and 88% in the contact force group (p=0.988). Squara et al reported a similar 1 -year freedom from recurrent paroxysmal AF (73% in the cryoballoon group and 76% in the contact force group (SmartTouch and Tacticath), p=0.63) in their ambidirectional (combined prospective and retrospective enrolment) multicentre cohort study of four participating centres (two centres performed both cryoballoon and RF ablation, one centre performed exclusively cryoballoon ablation and one performed exclusively RF ablation).30 Lastly, Ciconte et al reported a single-centre experience with 100 consecutive patients undergoing PVI for persistent AF with the second-generation cryoballoon (50 patients) and contact-force-guided irrigated RF ablation (SmartTouch and Tacticath, 50 patients).31 In this non-randomised study, the 1 -year freedom from recurrent AF (as detected by Holter monitoring at 1, 3, 6 and 12 months) was 60% in the cryoballoon group and 56% in the contact force group (p=0.78). Although none of these studies demonstrated a significant difference in the incidence of complications, a recent meta-analysis observed a lower incidence of pericardial effusion (OR 0.44, 95% CI 0.28 to 0.69, p<0.01) and tamponade (OR 0.31, 95% CI 0.15 to 0.64, p<0.01) with cryoballoon ablation in comparison with contact-force-guided RF ablation, whereas transient phrenic nerve palsy was more frequent after cryoballoon (OR 7.40, 95% CI 2.56 to 21.34, p<0.01).32
The multicentre, randomised FIRE and ICE trial was designed to determine whether cryoballoon ablation was non-inferior to RF ablation in symptomatic patients with drug-refractory paroxysmal AF.33 Patients were randomised to Arctic Front-based cryoballoon ablation (374 patients) versus irrigated RF ablation (376 patients). The primary efficacy end point (documented recurrence of AF/AT/AFL>30 s, AAD prescription or re-ablation) occurred in 138 patients in the cryoballoon group and in 143 in the RF group (1 year Kaplan–Meier event rate estimates, 34.6% and 35.9%, respectively; HR 0.96, 95% CI 0.76 to 1.22, p<0.001 for non-inferiority). However, despite reporting recently, it is important to note that the study was not an exclusive comparison of advanced technologies, with the majority of patients receiving non-contact-force irrigated RF ablation (284/376 in the RF group) and a significant proportion (90 of 374 patients) receiving first-generation cryoballoon ablation. As such, the relative safety and efficacy of these new technologies remains unknown.
Data supporting shorter freeze durations
The optimal duration of freezing, that is, how long the tissue should be kept in the frozen state, is not well established. Current recommendations are for cryoablation dosing at 240 s for each application, which is based on studies of an early focal cryocatheter. In these studies, it was observed that the effect of a cryoablation lesion reached a plateau of 3 min after the onset of ablation. Thereafter, ‘prolongation of exposure time beyond 3 min did not result in any further increase in lesion dimension or volume’.34 35 Since then, the cryocatheter has evolved from a rigid focal catheter to a semi-compliant balloon, which necessitated a redesign of the cryorefrigerant delivery mechanisms. Moreover, the refrigerant itself has changed from slow-cooling to more efficacious gases (ie, nitrous oxide).
Information regarding the safety and efficacy of shorter cryoballoon ablation durations is limited to 3 min lesions, which have been suggested in several non-randomised studies to be of comparable efficacy with longer duration cryolesions.36 37 We recently completed a randomised preclinical study examining the immediate and delayed effects of shorter ablation time on PVI efficacy.38 In our study, 32 mongrel dogs underwent cryoballoon ablation with a 23 mm cryoballoon catheter. PVI procedures were randomised to a single 2 min versus 4 min cryoballoon application. Although 4 min lesions were associated with a thicker neointima than 2 min lesions (223.8 vs 135.6 µm, p=0.007), no differences were observed in the rates of procedural PVI or the achievement of complete circumferentially transmural lesions at 30 days (78% overall, 86.2% for 2 min vs 70% for 4 min, p=0.285);, however, a reduction in late PV strictures was observed in the 2 min group (6/30 PVs with strictures in the 4 min freeze duration vs 0/29 PVs with strictures in the 2 min freeze duration, p=0.024).
Arrhythmia monitoring
Although from a patient perspective the freedom from symptoms related to AF may be the most important clinical end point, contemporary evidence suggests that there is a poor correlation between symptoms and AF burden.39 40 Moreover, the presence or absence of symptoms does not affect the prognosis and complications of the AF.41 As such, any evaluation of treatment efficacy must include protocol-determined arrhythmia monitoring. Given that paroxysmal AF is by definition a disease of clusters, studies have shown that the detection of AF recurrence is proportional to the duration of monitoring.42 Specifically, Kottkamp et al demonstrated an increased detection of arrhythmia recurrences post-AF ablation for highly symptomatic AF in a group undergoing serial 7 day ECG monitoring versus those undergoing only intermittent ECG monitoring (26% vs 12% documented recurrence).43 Unfortunately, although non-invasive intermittent rhythm monitoring remains the most widely used method of ascertaining ablation efficacy, it often fails to detect AF recurrence. Specifically, the sensitivity (31%–71%) and the negative predictive value (21%–39%) are significantly inferior to continuous monitoring techniques.44 This imprecision associated with intermittent arrhythmia monitoring confers a significant risk of Type II error, which makes it inappropriate for outcome ascertainment in a trial designed to evaluate the efficacy of different therapeutic interventions.
As such, a major strength of the current study is the reliance on continuous cardiac monitoring for the determination of arrhythmia outcomes. All participants in the CIRCA-DOSE trial will have an implantable cardiac monitor with an automated AF detection algorithm (REVEAL LINQ) inserted a minimum of 1 month prior to ablation. This subcutaneous implantable cardiac monitor continuously analyses the beat-to-beat variability of cardiac cycles leading to an accurate determination of the timing of arrhythmia recurrence, as well as an accurate quantification of AF burden (hours in AF per day and percentage of overall time in AF). With respect to this latter point, the use of AF burden allows for a more detailed examination of the relative efficacy of the three-different treatment approaches, beyond which can be obtained with dichotomous event analyses such as ‘time to first AF recurrence.’ Unfortunately, intermittent rhythm monitoring techniques are unable to accurately quantify AF burden.44
Methods and analysis
Study design
The CIRCA-DOSE study (ClinicalTrials.gov #NCT01913522) is a multicentre prospective randomised clinical trial. The study will be conducted at eight participating clinical centres in Canada. The protocol has been developed in accordance with SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) guidelines.
Participants
Patients aged >18 years with symptomatic paroxysmal and early persistent AF that is refractory to at least one AAD and who have been referred for initial percutaneous catheter ablation will be screened for eligibility. At least one episode of AF must be documented on 12-lead ECG, transtelephonic monitor or Holter monitor within 24 months of randomisation. Inclusion and exclusion criteria are detailed in online supplementary table 1.45
Screening and selection
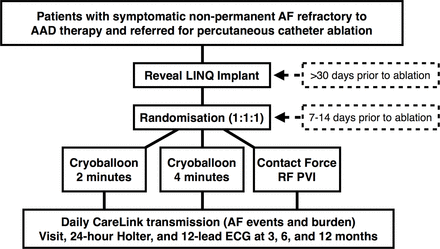
Patients referred for catheter ablation of symptomatic AAD-refractory AF and meeting the eligibility criteria will be offered the opportunity to participate in the trial (figure 1). Informed consent and baseline clinical data will be obtained by the physician investigator.
Study flow diagram. AAD, antiarrhythmic drugs; AF, atrial fibrillation; PVI, pulmonary vein isolation; RF, radio frequency.
Randomisation
Patients who meet eligibility criteria and give informed consent will be assigned in a 1:1:1 ratio using permuted block randomisation according to a computer-generated sequence, with a block size of 6 and 12 per site to (1) standard RF ablation guided by tissue-contact force, (2) short cryoballoon ablation duration (2 min cryoapplications) and (3) standard cryoballoon ablation duration (4 min cryoapplications). An independent, blinded statistician will generate the block randomisation scheme. Patients will be blinded to their randomisation assignment.
Loop recorder implant
Patients who meet eligibility criteria and give informed consent will undergo the implantation of an implantable cardiac monitor (ICM) a minimum of 30 days prior to the index ablation for the purpose of arrhythmia monitoring (Reveal LINQ, Medtronic). The ICM has an AF detection algorithm that analyses beat-to-beat variability of cardiac cycles on a 2 min ECG strip. Arrhythmia events meeting these criteria are stored for independent adjudication. The device is also capable of quantifying the amount of AF per day and the overall AF burden (percentage of the observed time that a patient is in AF). Additionally, the patient can activate the device manually to facilitate analysis of heart rhythm during symptomatic events. ICM programmed parameters are summarised in online supplementary table 2.
Catheter ablation procedure
Effective anticoagulation with oral vitamin K antagonists (target INR between 2 and 3), low molecular weight heparin or dabigatran/apixaban/rivaroxaban for at least 1 month and/or the exclusion of a left atrial (LA) thrombus by transoesophageal echocardiography (<48 hours preablation) is mandated prior to ablation.46 AADs will be discontinued five half-lives before the procedure, except for amiodarone, which will be discontinued 8 weeks prior to ablation. Interventions will be performed on patients in the fasting state under conscious sedation or general anaesthesia, per local practice.
For each of the three treatment arms, patients will undergo PVI according to standard clinical practice.46–49 No prophylactic LA linear ablation lesions or ablation of complex fractionated atrial electrograms will be permitted in addition to PV isolation. In the event of documented right atrial cavotricuspid isthmus dependent flutter, cavotricuspid isthmus ablation is permitted (with irrigated RF or focal cryoablation).
Contact-force-guided RF ablation
For patients randomised to RF catheter ablation a three-dimensional, non-fluoroscopic mapping system (CARTO3, Biosense Webster) will be used for anatomic reconstruction. Through one trans-septal access, a circular mapping catheter (CMC) (decapolar or duo-decapolar) will be advanced into the LA. The CMC will be placed sequentially within each PV to record baseline electrical activity (PV potentials; PVPs). Via a second trans-septal access, an irrigated-tip contact-force sensing RF ablation catheter (Thermocool SmartTouch or SmartTouch Surround Flow, Biosense Webster) will be positioned in the LA. Circumferential ablation lesions will be placed via the ablation catheter 1–2 cm from the PV ostia to electrically isolate the PV, as per standard practice.45 RF energy will be delivered at 20–35 W to a maximum temperature of 43°C. The contact force targeted prior to lesion delivery will be 20 g (acceptable range 10–40 g), with a minimum individual target lesion duration of 400 gram-seconds force–time integral. Circumferential lesions around the veins will be considered complete when the procedural end point has been reached (see Procedural Endpoint section below).
Cryoballoon ablation
For patients randomised to cryoballoon ablation, the trans-septal sheath will be exchanged over a guidewire with a steerable 15-Fr sheath (FlexCath, Medtronic). Before introducing the balloon catheter (Arctic Front Advance, Medtronic) in the sheath, a 15 or 20 mm diameter CMC will be inserted in the central lumen of the cryoballoon. A 23 or 28 mm cryoballoon will be advanced through the steerable sheath into the LA with the CMC used as a guidewire. Although the use of the larger (28 mm) cryoballoon is preferred, the 23 mm cryoballoon may be used based on physician judgement for PV diameters <20 mm.50–52 Before ablation, the CMC will be positioned in the venous ostium to record baseline electrical activity. The CMC will then be advanced more distally for support. The cryoballoon will be positioned in the venous ostium, and the degree of occlusion will be tested through the injection of 1:1 diluted contrast material. Vessel occlusion will be evaluated according to a semi-quantitative grading (see online supplementary table 3). Prior to ablation of right-sided PVs, a catheter will be placed in the superior vena cava cranial to the right superior PV in order to pace the right phrenic nerve (10–20 mA at 1.0–2.0 ms pulse width at a cycle length of 1000 ms). Ablation will be immediately terminated on any perceived reduction in the strength of diaphragmatic contraction or a 30% reduction in the diaphragmatic compound motor action potential amplitude as measured via diaphragmatic electromyography.53 If the procedure is performed under general anaesthesia, paralytic agents will be discontinued at least 30 min prior to phrenic nerve pacing.
Patients randomised to standard cryoballoon ablation will undergo cryoablation with a target duration of 4 min. Once PVI is achieved, a single additional application of 4 min cryoapplication will be delivered after the rewarming phase (to +20°C).
Patients randomised to short cryoballoon ablation will undergo cryoablation with a target duration of 2 min. Once PVI is achieved, a single additional 2 min cryoapplication will be delivered after the rewarming phase (to +20°C).
Ineffectual cryolesions
Excepting common ostia, cryoablation lesions that fail to isolate the vein (if real-time PV potential monitoring is feasible) or fail to achieve a temperature colder than −35°C after 60 s of ablation onset will be considered ineffectual and be terminated. Thereafter, the balloon and/or guidewire should be repositioned and a new lesion delivered.
Inability to isolate
Should the operator fail to isolate the PV (excluding common ostia) after a minimum of three attempted cryoballoon applications, then focal ablation with the 8 mm cryocatheter (Freezor Max) targeted to sites of LA-PV breakthrough will be permitted at operator discretion.
Procedural end point
For all three treatment arms, the ablation procedure will be considered successful when PVI, as confirmed by bidirectional conduction block between PV and LA, has been achieved in accordance with the 2012 HRS/EHRA/ECAS consensus document.45 Bidirectional conduction block is defined as the combination of entrance block (the stable absence of conduction into the PV from the LA) and exit block (the stable absence of conduction from the PV into the LA, either spontaneous or during pacing from the CMC positioned at the PV ostium). Patients remaining in AF at the end of the procedure will be electrically or chemically cardioverted back to sinus rhythm. Remapping of all PVs post-cardioversion will be performed to the procedural end point.
Evaluation of spontaneous reconnection and dormant conduction
For all three treatment arms, a 20 min observation period (beginning at the end of the last ablation lesion) will be used to assess spontaneous recovery of conduction.45 If spontaneous reconnection occurs, the reconnected PVs will be re-isolated according to the randomised protocol.
Dormant conduction will be assessed with the use of a circular catheter in each PV by intravenous injection of 6 mg or more of adenosine to obtain at least one blocked P wave or a sinus pause ≥3 s. Dormant conduction will be defined by reappearance of PV conduction for ≥1 beat. If there is no dormant conduction in any PV, then the procedure will be considered complete. If dormant conduction is elicited, the patient will undergo additional targeted ablation according to the randomised protocol until dormant conduction is abolished (ie, adenosine fails to induce reconnection in any PV).
Post-ablation follow-up
Barring complications, patients will be discharged within 24 hours after the ablation procedure. Scheduled follow-up visits will occur at 3, 6 and 12 months from the first ablation procedure (within a 2 week margin; table 1). A 24-hour Holter and a 12-lead ECG will be performed at 3, 6 and 12 months. Automatic transmissions from the ICM will be obtained on a daily basis via CareLink. Patients will be instructed to record symptomatic episodes via use of the patient activator.
Schedule of enrolment, interventions and assessments
All patients will remain anticoagulated for ≥3 months postprocedure. Although discontinuation of oral anticoagulation during the study period is strongly discouraged, in patients with a CHA2DS2VASc score of <2, aspirin alone may be considered at treating physician discretion. Arrhythmia recurrence during the first 3 months postablation may be treated with cardioversion and/or AADs (except amiodarone). Where possible, repeat ablation procedures will be deferred until after the 3-month blanking period due to the potential for delayed cure (as per standard practice and in accordance with HRS/ECAS/EHRA recommendations).45 If AADs (except amiodarone) are used in the first 3 months postablation, they will be discontinued five half-lives before the end of the 3-month blanking period.45
Study outcomes
Primary end point
It is the time to first recurrence of symptomatic or asymptomatic AF, atrial flutter or atrial tachycardia (AF/AFL/AT) documented by 12-lead ECG, surface ECG rhythm strips, 24-hour ambulatory ECG (Holter) monitor or on ICM between days 91 and 365 post-ablation, or a repeat ablation procedure between days 0 and 365 post-ablation. AF or atrial flutter/tachycardia will qualify as an arrhythmia recurrence after ablation if it lasts 30 s or longer (on surface ECG rhythm strips, 24-hour ambulatory Holter monitor) or 120 s or longer on ICM (the minimum programmable episode interval). All tracings will be independently adjudicated by a committee blinded to treatment allocation. The primary end point and the 3-month blanking period adhere to the Heart Rhythm Society recommendations for reporting outcomes in AF ablation trials.45
Secondary end points
These are listed in online supplementary table 4.
Event adjudication
The clinical events committee (CEC) will be composed of a cardiac electrophysiologist as chairperson and six cardiologist reviewers with expertise in clinical event adjudication. Two reviewers will be assigned to review each end point and serious adverse event (SAE) with disagreement resolved by the chairperson or entire CEC (as outlined below).
Sample size
The sample size was determined based on the primary end points for the two main comparisons of interest: cryoablation with a 4 min application versus contact-force-guided RF catheter ablation and cryoablation with a 2 min application versus RF catheter ablation. Overall event-free survival at 1 year is estimated to be 65%. With a sample size of 111 per group and a two-sided 0.025 significance level (to account for the two main comparisons), the study will have 80% power (using a log-rank test) to detect a relative difference of 20% between contact force RF catheter ablation and either of the two cryoballoon ablation groups. Factoring in a 4% loss to follow-up, 116 patients per group should be randomised, for a total study population of 348. Power calculations are based on the log-rank test for equality of survival curves (nQuery, V.6.01), using simulated data.
Data management
A unique subject number not derived from personal identifiers will be used for subject identification. Study information using this unique subject number will be collected using case report forms, which will be entered into a secure online platform (InForm V.6.0). All electronic data are encrypted, password protected and stored on a secure network within the coordinating centre. The coordinating centre will perform regular evaluations of data integration and quality, management and resolution of data discrepancies, tracking of adverse event information, database quality control, and generate reports for principal and co-applicants, study sites and for committee meetings. At the conclusion of the study, the coordinating centre will lock the clinical data and perform the final analysis of the trial results.
Statistical analyses
Analysis of the primary and secondary end points will be based on the intention-to-treat principle according to the initial allocated strategy. Survival curves will be estimated by the Kaplan-Meier method and compared by the log-rank test. The two main comparisons will be cryoablation with a 4 min application versus RF catheter ablation, and cryoablation with a 2 min application versus RF catheter ablation. The comparison between the two cryoablation groups will be considered secondary.
A Cox proportional hazards model will also be used to test the consistency of the group effect while accounting for clinically important baseline characteristics, which will include ablation site, age, gender, race, weight, LA size, structural heart disease, AF duration and number of AADs used in the past. The proportional hazard assumption will be assessed by visual inspection of the log-negative log plot and through a formal test of the interaction term ‘group x time’ at α =0.05. Should this assumption fail, a stratified Cox model will be fitted in order to correct for non-proportional hazards if possible, or if ineffective, time-dependent variables will be introduced. Should these corrective techniques fail, logistic regression will be used instead.
Secondary end points expressed as time to event will be analysed similarly using Kaplan-Meier survival curves and a log-rank test. For all dichotomous qualitative variables, χ2 tests will be performed to assess group differences. Continuous variables, such as arrhythmia burden, will be analysed using an analysis of variance. If the data are not normally distributed, then the non-parametric Wilcoxon signed rank test will be used. Health-related quality-of-life scores will be compared by analysis of covariance, adjusting for baseline values to reduce the error mean squares. In the event of missing data, a multiple imputation approach using SAS procedures PROC MI and PROC MIANALYZE will be considered. All tests will be conducted at an alpha level of 0.05 with the exception of the two main comparisons that will be conducted at an alpha level of 0.025. Similarly, HRs for these two comparisons will be presented with 97.5% CI.
Data monitoring and CEC
A seven-member CEC will be composed of a cardiac electrophysiologist as chairperson and six cardiologist reviewers with expertise in clinical event adjudication. The CEC members are independent from the sponsor and investigators, are blinded to the study allocation and have no conflicts of interest relevant to the trial. The CEC is responsible for review and adjudication of all primary and secondary arrhythmia end points, which include SAEs and major complications. Information about the occurrence of any SAE is sought at all scheduled visits. For all adverse events, source documentation will be obtained prior to CEC review. Two reviewers will be assigned to review each end point and SAE. If both reviewers agree, the chairperson will be provided with the reviewer’s forms, and he will ratify the adjudication by completing the final adjudication form. If the reviewers are in disagreement, the chairperson will review the event and will serve as the third reviewer. If there is still disagreement between all three reviewers, a meeting will be scheduled to discuss the event.
Ethics and dissemination
Enrolment in the trial is predicated on the assumption that patients have already made the decision to undergo a catheter ablation procedure for drug-refractory AF. The catheter ablation procedure used in this study is the same as the standard treatment method for AF and is not experimental. The risks of participation are therefore the same as those of standard AF ablation, and independent of trial enrolment, participants in the study would have accepted these risks. Full ethics approval has already been obtained at all the participating sites.
The dissemination plan for the trial encompasses multiple modalities and strategies, including an integrated and an end-of-project knowledge-translation (KT) strategy. The integrated approach of the programme benefits from the involvement of non-profit organisations with a mandate of end user engagement and education (the Heart and Stroke Foundation of Canada), patients (the end user) and healthcare professionals. The involvement of these groups from the planning phase to completion represents an optimal strategy for engagement and empowerment, essentially creating invested champions at each level. Post-project KT will leverage the involvement of these groups to optimise the ability to reach the end users. The information derived herein (whether positive or negative) will be disseminated through established channels such as peer-reviewed publication, national and international meetings, webinars and through social media. The involvement of Medtronic CryoCath will facilitate the dissemination of the findings to end users through their established educational infrastructure (Medtronic Academy website, ‘user meetings’, to small group interactive conferences through their clinical specialist network). The end result of this KT plan will be the delivery of the optimal tailored treatment strategy to the individual patient at the optimal time.
References
Footnotes
Contributors JGA, MD, PK, AT and GAW conceived the study. JGA, AT, AV, LM, PK, MD, MWD and GAW were involved in the drafting of the peer-reviewed grant, the study protocol and the manuscript. MB, JC, PL-S, PN, J-FR, JS and AV served on the steering committee for the trial and have provided critical revision of the manuscript.
Funding The CIRCA-DOSE study was funded by a peer-reviewed grant from the Heart and Stroke Foundation of Canada (grant no G-13-0003121), with additional financial support from Medtronic and Heart Rhythm Services at the University of British Columbia. JGA and MWD are supported by a Michael Smith Foundation Clinical Scholar award (MSFHR 5963, and 5967). JGA, JC, MWD, PL-S, J-FR, AT, AV and GAW are Network Investigators of the Cardiac Arrhythmia Network of Canada (CANet), a Networks of Centres of Excellence.
Competing interests JGA reports grants and personal fees from Medtronic, grants from Baylis and personal fees from Biosense. AV reports grants and personal fees from Medtronic and Biosense. MWD reports grants from Biosense-Webster. AT has nothing to disclose. PL-S reports personal fees from Medtronic, personal fees from Johnson & Johnson and Biosense Webster. J-FR reports personal fees from Biosense Webster, Bayer, Servier, BMS-Pfizer and Boehringer. MD reports grants and personal fees from Medtronic. JS reports grants from Biosense Webster, personal fees from Biosense Webster, grants from St Jude Medical, personal fees from St Jude Medical and personal fees from Medtronic. LM reports personal fees from Medtronic and grants and personal fees from Biosense-Webster.
Ethics approval University of British Columbia Office of Research (Services) Ethics Clinical Research Ethics Board, as well as the local institutional review boards for each of the participating sites.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement At this point, there is no unpublished data as it is a study protocol.