Article Text
Abstract
Introduction Breast-cancer-related upper extremity lymphoedema (BCUL), a common complication of mastectomy, can cause physical discomfort, psychological distress, cosmetic defects, functional disability and chronic recurrent erysipelas in the affected arm(s). It is a challenge to physicians involved in the management of these patients. Wuling San, a classic prescription in Traditional Chinese Medicine used in treating oedema for thousands of years, is reported by many Chinese journals to perform well in BCUL. Therefore, the aim of this study is to verify its efficacy and evaluate its safety using rigorous methodological designs in patients with BCUL.
Methods and analysis To verify the efficacy and assess the safety of Wuling San over a placebo, this double-blind, randomised, placebo-controlled, multicentre trial will be carried out in three hospitals. A total of 200 eligible patients with BCUL will be randomly allocated, in a ratio of 1:1, to either the experimental medicine group or the placebo group. The primary outcome measure will be the proportion of absolute reduced limb volume, as measured by perometry. The second outcome measure will be the number of participants with adverse events. The assessment will be carried out at the following time points: before enrolment (baseline) and 2, 4, 6 and 8 weeks after treatment.
Ethics and dissemination This trial will be conducted in accordance with the Declaration of Helsinki and supervised by the institutional review board of the Fourth Affiliated Hospital of Guangxi Medical University (approval number PJK2016088). All patients will receive information about the trial in verbal and written forms and will give informed consent before enrolment. This trial will help to demonstrate whether Wuling San is effective in the treatment of patients with BCUL. The results will be published in peer-reviewed journals or disseminated through conference presentations.
Trial registration number NCT02726477; Pre-results.
- Wuling San
- lymphedema
- protocol
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Strengths and limitations of this study
This is the first clinical study focusing on the efficacy and safety of the use of Wuling San for breast-cancer-related upper extremity lymphoedema (BCUL).
This study should provide a reference for future large-scale studies if applicable.
Subsequent studies will be considered for comparisons between treatment with Wuling San and other therapies in patients with BCUL, if it is proved efficacious.
Background
As a common symptom caused by insufficient lymph drainage following radical mastectomy or modified radical mastectomy, breast-cancer-related upper extremity lymphoedema (BCUL) afflicts over 30% of postoperative patients who undergo axillary lymph node dissection (ALND).1 Based on the severity, which depends on the extent of the axillary operation, the frequency of radiotherapy, lymph node status, tumour burden, age of the patients and obesity,2 ,3 BCUL can be classified into three stages. Stage I is characterised by a ‘reversible’ protein-rich fluid accumulation. Stage II, known as ‘spontaneously irreversible’, is characterised by an increased number of keratinocytes and connective tissue cells. Stage III, also called ‘elephantiasis’, is characterised by a conspicuous increase in the size of the limb, with thickened rough skin due to massive hyperkeratosis.4 This can result in physical discomfort, psychological distress, functional disability, cosmetic defect and chronic recurrent erysipelas.5 Affected arms are often stiff and prone to infections, which often require hospitalisation for antibiotics,6 or even surgery,7 a significant source of expenditure and hardship for many patients. To prevent deterioration from the swelling, patients are repeatedly obliged to wear uncomfortable elastic compression stockings, which serve as a continuous reminder of their cancerous status, increasing their anxiety and reducing their quality of life.8 However, as a poorly understood symptom with few effective therapies, the management of BCUL is still a challenge for physicians.9
A basic concept in the theory of Traditional Chinese Medicine (TCM) is that related symptoms of certain diseases are often summarised as a syndrome (‘zheng’ in Chinese).10 In TCM, lymphoedema is put into the same category as oedema and is considered to be caused by a qi (chi) deficiency and blood stasis. Several prescriptions are available to manage this symptom, and Wuling San is one of the most popular. Wuling San is composed of extracts from Poria, Rhizoma Alismatis, Polyporus, Cortex Cinnamomi and Rhizoma Atractylodis Macrocephalae. With the ability to promote qi transformation, warm yang and drain dampness, this formula is from the Treatise on febrile diseases caused by cold (Shang Han Lun) written by Zhongjing Zhang during the Chinese Eastern Han Dynasty (about 200 AD), and has been used for thousands of years since then in East Asia.11–13
Both the early clinical reports in Chinese journals14–17 and the clinical data gathered by the physicians in our team have shown that Wuling San somehow decreases swelling and relieves the symptoms of lymphoedema in the upper extremities after ALND, probably by promoting kidney function, diuresis, and other pathways. But these are only experience-based medical accounts with little scientific experimental evidence. Therefore, the aim of this trial is to check its efficacy and assess its safety in the treatment of women with modest lymphoedema (stage I or II) after mastectomy for breast cancer.
Method and analysis
The present protocol was designed according to Standard Protocol Items: Recommendations for Interventional Trials 2013 (SPIRIT 2013; see online supplementary file S1).
supplementary file
Study design
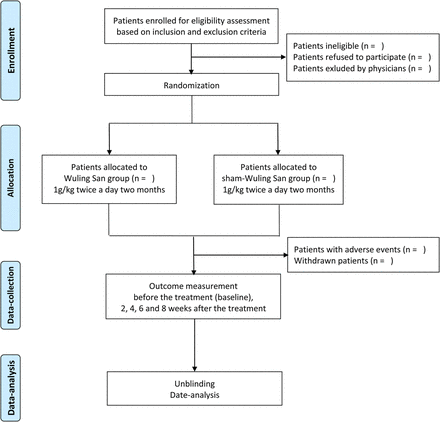
This double-blind trial will be carried out in three medical centres: the Fourth Affiliated Hospital of Guangxi Medical University, Liuzhou Maternity and Child Healthcare Hospital and the Third Affiliated Hospital of Guangxi University of Chinese Medicine. The trial will be conducted from September 2016 to June 2018. A total of 200 patients will be recruited, and for each participant a standard treatment will be performed with the same dosage and duration. The flow chart of this trial is outlined in figure 1.
Flow chart.
Recruitment of participants
The coordinators with the assistance of physicians in each centre, will identify eligible participants preliminarily based on the inclusion and exclusion criteria. Their eligibility will then be confirmed by the physicians in charge after a baseline evaluation. Written informed consent will be obtained from every patient before enrolment.
Inclusion and exclusion criteria
The following inclusion criteria will be adopted in this study: women aged 20–40 with unilateral BCUL, stage I or II (mild to moderate lymphoedema with a minimum 10% and maximum 40% increase in volume compared with the unaffected arm based on perometry evaluation); body mass index 18–25; who have completed all primary and adjuvant treatments (surgery, chemotherapy, radiotherapy) without any evidence of breast cancer recurrence. Patients who meet the following criteria will be excluded: bilateral or stage III lymphoedema; history of bilateral ALND; recent history of cellulitis in the affected extremity (within the last 3 months); recurrent breast cancer or other malignancies; current use of chemotherapy for breast or other malignancies (within the last 30 days); current use of radiation for breast or other malignancies (within the last 3 months); pregnant or lactating women; patients who are taking diuretic drugs such as diosmin or other investigational drugs at the time of enrolment (within the last 30 days); serious renal or cardiac failure; unable to comply with the protocol or the measurement schedule.
Loss to follow-up and data management
Patients may drop out of the trial at any time for any reason. If a patient intends to drop out, the physician in charge should ask for the exact reason and give proper advice and assistance. Once a patient decides to withdraw, data on lymphoedema will immediately be collected and the duration of administration will be recorded. Patients will be able to withdraw from the study on the basis of disease progression or serious complications. Incidences of loss to follow-up will be reported to the chief designer of this trial every month. Data from the trial will be collected in case report forms using the double entry method. The coordinator in each centre will check each completed report once a month to ensure the quality of the case report form.
Forbidden drugs and concomitant treatments
This trial is not compatible with any of the following interventions: diuretics, diosmin and hesperidin. Other concomitant treatments should be discussed with the physician in charge and carefully recorded.
Randomisation and blinding
According to the inclusion and exclusion criteria, eligible patients will receive a standard interview and receive more information about the trial. After informed consent has been obtained and a baseline evaluation performed, patients will be randomised to the experimental or placebo group in a ratio of 1:1. As it is a double-blind trial, randomisation is performed by the chief designer. The randomisation sequence (blocked, stratified for centres) will be generated using the RAND function in Microsoft Excel (see online supplementary file S2). Each centre will receive a set amount of drug/placebo in envelopes with consecutively coded labels. All of the drugs (including the placebo) will be provided by the pharmacy department of the Fourth Affiliated Hospital of Guangxi Medical University, and will be labelled by the chief designer according to the randomisation sequence and given to the coordinators. Emergency letters on each coded drug will be sent to each centre and properly safeguarded in case of any possible serious adverse event. Treatment assignments will be kept from both patients and investigators (including statisticians) until the study is completed.
supplementary file
Intervention
Experimental group
Patients in the experimental group will be given Wuling San dissolved in warm water at a dosage of 1 g/kg twice a day for 8 weeks. The ingredients (purchased from the China National Pharmaceutical Group Corporation) are listed in table 1. These medical herbs will be made into powder at the pharmacy department of the Fourth Affiliated Hospital of Guangxi Medical University, using a herbal pulveriser by experienced and licensed TCM doctors qualified for at least 3 years. The powder will be sifted through a sieve of 150 μm.
Prescription of Wuling San
Control group
Patients in the control group will be given placebo powder with the same dosage, frequency and duration. The placebo powder, similar to Wuling San in colour, smell, taste and packaging, is composed of 5% Wuling San, 93% starch and 2% food colouring. All the test drug packages will be tagged with the name of the patients by coordinators in each centre and dispensed by nurses in our team, who will be responsible for recording any incidents such as reactions to the drug, symptoms related to the drug and concomitant treatments during the trial.
In addition to the Wuling San treatment, both groups will receive standard decongestive therapy, consisting of daily manual lymph drainage, gentle exercise and elastic compression garments. All these steps will be implemented for a fixed duration of 2 hours a day and 5 days a week. Otherwise, detailed records will be made.
Outcome measurements
For every patient, measurement of both limbs by perometry will be carried out at the following time points: before treatment (baseline), 2, 4, 6 and 8 weeks after treatment (table 2). After the limb volumes have been collected for each patient, the proportion of absolute reduced limb volume after the therapy will be calculated using the following formula adapted from Andersen et al18
Summary of measurements conducted in this trial at all time points
VL is the volume of the affected arm with lymphoedema; VC is the volume of the contralateral arm; VLB is the volume of the affected arm with lymphoedema measured before treatment; VCB is the volume of the contralateral arm measured before treatment; VLA is the volume of the affected arm with lymphoedema measured after treatment; VCA is the volume of the contralateral arm measured after treatment.
Data such as measurements collected at weeks 2, 4 and 6 after treatment will also be recorded for monitoring and interim analyses. In the case of withdrawn patients, the assessment will be performed before the withdrawal. The number of participants with serious and non-serious adverse events will serve as the secondary outcome measure.
Safety outcomes
Routine tests, such as urine test, stool test, blood test, liver function test, renal function test and ECG, will be carried out for each patient every week during the treatment.
Adverse events
Every adverse event will be recorded in detail and closely monitored before stabilisation or resolution. The chief designer of this trial and the corresponding coordinator will be informed immediately unless it has been confirmed that it was not caused by the treatment. They will cooperate with the physician in charge to evaluate the severity and determine the causality of the events. All relevant adverse events will be reported to the institutional review board of the Fourth Affiliated Hospital of Guangxi Medical University within the relevant time frames. The chief designer of the trial will be responsible for reporting all adverse events. The coordinators will be responsible for establishing the standard procedures and the training of relevant staff before trial initiation. Regular monitoring will be used to ensure that all adverse events are identified and addressed appropriately.
Sample size calculation
To the best of our knowledge, no multicentred, randomised, placebo-controlled trial investigating the efficacy of Wuling San for BCUL has been conducted. Therefore, there is no reference for the sample size needed in this trial. However, to perform this pilot trial and collect adequate data, we hypothesise the effect size, risk of type 1 error (α) and risk of type 2 error (β) to be 0.25 (medium), 0.05 and 0.2, respectively, based on the recommendation from the manual of G-Power (V.3.1). Using the F test and choosing ‘ANOVA fixed effects omnibus one way’ and ‘A Priori compute required sample size given α, power and effect size’, then 159 is the minimum required sample size. If the estimated dropout rate is set at 15%, at least 183 patients should be recruited for this trial (see online supplementary file S3). We intend to include 200 patients, 100 for each arm.
supplementary file
Data analysis
An independent statistician will be invited to perform the data analysis, made up of a per-protocol (PP) analysis and an intent-to-treat (ITT) analysis. The PP analysis will be restricted to participants who strictly follow the protocol and complete the study. In contrast, the ITT analysis will include all the patients who have ever received any treatment in this trial, where missing data will be supplemented using the LOCF (last observation carried forward) method. Mean and SD will be used for continuous variables, and percentages for categorical variables. One-way analysis of variance will be used to compare the treatment groups with the placebo group and to analyse the possible blocking factor due to the multicentre design. Covariance adjusted for clinical centre and baseline will also be used in the analysis if significant differences are found between centres. Interim analyses, using the data collected during the trial, will be performed by the Data and Safety Monitoring Board (DSMB) so as to have active monitoring on the ongoing status of the trial and to make sure the trial is not continued simply because it has begun. Statistical analyses will be performed using SPSS (V.16.0). For all comparisons, p<0.05 will be considered significant.
Trial oversight
Three groups will be responsible for the supervision of this trial.
The Clinical Trial Steering Committee (CTSC)
The CTSC is composed of the chief trial designer and coordinators at each centre, who will have a meeting every month to review and address key technical and operational problems.
The Coordinating Committee
The Coordinating Committee, composed of local research members in each centre, will work closely with the CTSC to coordinate trial activities and will be responsible for the management of trial sites, preservation and distribution of trial materials, dissemination of information from the trial, collection, organisation and preservation of data and documents, safety reports, electronic case report forms, and training of trial staff.
DSMB
The DSMB, serving as an independent third party, is composed of experienced and knowledgeable experts. It will be responsible for periodical review and data evaluation for participants' safety and trial progress, and make recommendations to the CTSC regarding the modification or termination of the trial. The board members will meet every month.
Discussion
In recent years, instead of ALND, sentinel lymph node biopsy (SLNB) has become a more common procedure in breast cancer surgery, which has reduced the incidence of BCUL.19 However, a certain proportion of patients still develop arm lymphoedema, especially when SLNB is combined with axillary radiotherapy.20 Various conservative therapies, such as physical therapy, lymphatic drainage, massage, acupuncture, pneumatic pumps, oral pharmaceuticals, laser therapy, compression garments, limb exercises and psychological support, have been applied in clinical practice for patients with BCUL. However, investigations and comparisons of these therapies performed in two systematic reviews21 ,22 found it difficult to make definitive therapy recommendations. Moreover, most therapies were shown to be merely more beneficial than doing nothing at all for the lymphoedematous limb. Therefore, there is still an urgent need to find an effective and convenient therapy for those patients who go on to develop BCUL after breast cancer treatment.20 If this therapy is economically feasible, it will be more beneficial to patients, because of the chronic process of BCUL.23
In TCM, lymphoedema including BCUL is classified as a kind of oedema, which is considered to be a syndrome of internal stagnation of fluid dampness due to stasis of the blood and a deficiency of the qi, and the treatment indication is Wuling San. Identified as a prescription for strengthening the yang qi and promoting water metabolism in organs such as the kidneys and bladder, Wuling San manages qi stagnation and blood stasis. Of the five ingredients of the Wuling San prescription, Polyporus Poria and Alismatis rhizome are the main ones with the ability to induce diuresis and eliminate dampness; Rhizoma Atractylodis Macrocephalae is considered to enhance the efficacy of Poria, and Cortex Cinnamomi is considered to strengthen the yang qi to promote water metabolism in organs such as the kidneys and bladder. Together, these five herbs endow Wuling San with the ability to restore the balance between yin and yang, promote water metabolism, and reduce stasis.
Some scientific reports have demonstrated that Wuling San can promote kidney function and induce diuresis.24 ,25 A clinical study reported that the use of Wuling San could lead to a significant increase in urine volume and a reduction in body weight in patients with nephritic syndrome.26 In addition, there are reports that Wuling San can inhibit the activity of the renal renin–angiotensin system in normal rats27 and in rats with adriamycin-induced nephrotic syndrome,28 thereby protecting the podocytes from injury. The efficacy of Wuling San in renal oedema has been observed in patients with diabetic nephropathy.29
Similar efficacies of the five ingredients of Wuling San have also been reported. Poria has been found to attenuate hypertonicity-induced cell death in the collecting tubule30 and ameliorate chronic kidney diseases by intervening in certain metabolic pathways in rats.31 A similar effect was found in patients with chronic kidney disease.32 Polyporus aqueous extract has been reported to have significant diuretic activity in rats, with a reduced level of aquaporin and an increased output of urinary Na+, K+ and Cl−.33 In addition, aqueous extract of Alismatis rhizome has been found to promote diuresis in rats34 and to have the ability to remove urinary calculi in patients with ureter calculi.35 Cortex Cinnamomi has been found to improve renal function effectively in mice.36 Furthermore, the bioactive components or extracts of the Atractylodis macrocephalae rhizome have been reported to enhance immunity in aged rats.37 In summary, these reports indicate that Wuling San might have diuretic efficacy and renal protective effects by enhancing kidney function and inhibiting stress response.
As it was greatly valued by their predecessors, the Chinese have used Wuling San in the treatment of oedema for thousands of years.11–13 In recent years, there have been several case reports in Chinese journals on the good clinical performance of Wuling San in the treatment of lymphoedema.14–17 Based on our clinical observation in practice, women with SCUL in stage I or II show a significant reduction in the size of the affected limb after Wuling San treatment in combination with standard routine management composed of daily use of compression stockings, exercise and skin care. Increased vitality and quality of life and fewer adverse events were reported by women receiving Wuling San, compared with those receiving other therapies. Moreover, the ingredients are inexpensive and natural, with few side effects.
However, no scientific clinical trial has confirmed the efficacy of Wuling San in the treatment of BCUL. Therefore, to maximally exclude the placebo effects and to demonstrate whether Wuling San is effective for treating BCUL, we designed this methodologically rigorous, randomised, double-blind trial. Sham-Wuling San can be used for blinding purposes because of the rare experience of Wuling San among patients with BCUL. In previous studies, no examples have used Sham-Wuling San. For practical purposes, we plan to use starch mixed with 5% Wuling San and 2% food colouring as a sham powder. A detailed description of our methods for recruitment, randomisation, allocation, intervention and data analysis is provided above.
However, there are also some limitations to this protocol. First, owing to limited studies in this field, the primary focus of this trial is mainly on the verification of its efficacy and the investigation of its safety; no further research is involved. Second, subsequent studies will be needed for other assessments, including comparisons between Wuling San and other therapies in patients with BCUL, if it is proved efficacious.
In conclusion, this pilot trial will verify the efficacy and investigate the safety of Wuling San in the treatment of BCUL, assess the feasibility of the study design, and provide a clinical foundation for future large-scale studies (if applicable).
Acknowledgments
We are grateful to Xuexiang Liu for help with statistical analysis.
References
Footnotes
HZ and ZP are the co-first authors
Contributors SD is the chief designer of this trial. HZ and ZP drafted the manuscript. ZP, HZ and XL are the coordinators and responsible for the screening and enrolment of patients. MD, YZ, LS, JC, BH, and FQ are involved in the recruitment of patients. SD is responsible for supervision of the study and revision of the manuscript. All authors read and approved the final version of the manuscript.
Funding This study was supported by Key Laboratory Construction of Tumor Diseases Prevention in Liu Zhou, Guang Xi (No (2014G020403)).
Competing interests None declared.
Patient consent Obtained.
Ethics approval Institutional review board of the Fourth Affiliated Hospital of Guangxi Medical University.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement The results of this pilot study will be disseminated via peer-reviewed publications and conference presentations. All of the data are available.