Article Text
Abstract
Objective To assess the frequency of warfarin use, the achieved international normalised ratio (INR) balance among warfarin users and the primary healthcare outpatient costs of patients with atrial fibrillation (AF).
Design Retrospective, non-interventional registry study.
Setting Primary healthcare.
Participants All patients with AF (n=2746) treated in one Finnish health centre between October 2010 and March 2012.
Methods Data on healthcare resource use, warfarin use, individually defined target INR range and INR test results were collected from the primary healthcare database for patients with AF diagnosis. The analysed dataset consisted of a 1-year follow-up. Warfarin treatment balance was estimated with the proportion of time spent in the therapeutic INR range (TTR). The cost of used healthcare resources was valued separately with national and service provider unit costs to estimate the total outpatient treatment costs. The factors potentially impacting the treatment costs were assessed with a generalised linear regression model.
Results Approximately 50% of the patients with AF with CHADS-VASc ≥1 used warfarin. The average TTR was 65.2% but increased to 74.5% among patients using warfarin continuously (ie, without gaps exceeding 56 days between successive INR tests) during follow-up. One-third of the patients had a TTR of below 60%. The average outpatient costs in the patient cohort were €314.44 with the national unit costs and €560.26 with the service provider unit costs. The costs among warfarin users were, on average, €524.11 or €939.54 higher compared with the costs among non-users, depending on the used unit costs. A higher TTR was associated with lower outpatient costs.
Conclusions The patients in the study centre using warfarin were, on average, well controlled on warfarin, yet one-third of patients had a TTR of below 60%.
- Primary Care
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/
Statistics from Altmetric.com
Strengths and limitations of this study
-
The study assesses age-specific and gender-specific prevalence of atrial fibrillation (AF), real-life warfarin use, treatment quality and costs among all patients with AF within one Finnish municipality.
-
The generalisability of the findings may be limited: warfarin use may differ in other countries and even in other Finnish municipalities.
-
The average primary healthcare outpatient costs in the studied municipality are among the lowest of average sized Finnish municipalities, suggesting that the costs presented in this study may underestimate the average cost of AF treatment in Finland.
Introduction
Atrial fibrillation (AF) is the most common form of cardiac arrhythmia. Its prevalence increases with age and has been estimated to be around 1%.1 ,2 As AF increases the risk of thromboembolic complications, Finnish treatment guidelines recommend oral anticoagulation treatment for patients whose CHA2DS2-VASc score is at least 1, and whose risk of stroke equals or exceeds 1% per annum.3 For decades the main alternative for anticoagulation treatment has been warfarin. Warfarin has a narrow therapeutic window and requires frequent monitoring to maintain the desired treatment targets. As a result, warfarin treatment places a burden on the healthcare sector, especially in areas with ageing populations.
In recent years, new oral anticoagulation (NOAC) alternatives have been developed.4–6 These NOACs do not require the same degree of monitoring as warfarin. As NOACs are more expensive than warfarin, a few studies have been published to assess whether the new anticoagulants can be considered to be cost-effective treatment alternatives to warfarin.7–9 In this regard, the costs associated with warfarin monitoring are of high importance. However, the published cost estimates vary widely from approximately €200 to €1900 per year.10–13 In addition, many authors have pointed out that the cost-effectiveness of new anticoagulants depends on the quality of the warfarin treatment.14–16
Our study aims to provide an overview of the anticoagulation treatment of patients with AF in one Finnish municipality (Joensuu) based on comprehensive primary care registry data. The primary aims were to assess the quality of warfarin treatment (based on individually specified target ranges for international normalised ratio (INR) when available) and the costs associated with treatment. In addition, the prevalence of AF in the municipality is estimated.
Materials and methods
The study was performed as an unrestricted, non-interventional retrospective database study in the municipality of Joensuu in Eastern Finland, which has 73 305 inhabitants,17 at the beginning of 2011. At the study centre, the blood sample for INR tests was obtained with two methods, venous blood draw or fingerstick. Venous draws were taken during scheduled appointments (preferably on Tuesdays or Thursdays 10:00–11:00) at the laboratory. The results and dosing guidance to patients receiving laboratory results were given alternatively by text messaging, phone (the nurse called the patient or vice versa) or in writing (the patient visited the health centre reception and the nurse marked the dose and time of the next INR test on the patient's ‘warfarin card’). Fingerstick samples were analysed with point-of-care devices, and the INR test results, as well as guidance related to doses, were given during the same scheduled appointment with a nurse. The preferred method for the warfarin monitoring at the study centre consisted of performing the INR tests using venous blood samples and informing the patients with a text messaging service. Patients needing special support in their warfarin treatment or those who were unable to use text messaging services and who had stable INR values were eligible for fingerstick measurements. The equivalence of fingerstick and venous sample-based INR results was confirmed with simultaneous venous and fingerstick measurements for the first three fingerstick measurements at the start of treatment and at every seventh test thereafter.
All patients with an AF (International Classification of Diseases, 10th Revision (ICD-10) code I48) diagnosis were included in the study cohort. The following information was gathered from the electronic primary care database (Mediatri patient information system): age, gender, comorbidities, date of death (if the patient had died by June 2012), individually defined INR target range, INR test results, use of antithrombotic medicines, physician visits, physician phone consultations, nurse visits, nurse phone consultations, primary care inpatient stays and use of home nursing services. The data on physician visits, physician phone consultations, nurse visits, nurse phone consultations and inpatient stays included the dates for service use, and ICD-10 and/or International Classification of Primary Care (ICPC) codes, if the codes had been marked by the personnel. The data were collected directly from the primary care databases in numerical format in June 2012, which covered the time period of 1 October 2010 to 31 March 2012. The primary care database covers practically all diagnosed patients in the municipality.
The analysed dataset, consisting of a 1-year follow-up, was formed as follows: start of an individual's follow-up was defined as the date when healthcare services were first used (any of the following reasons: INR test, physician/nurse visit, physician/nurse phone consultation and inpatient stay) closest to 1 January 2011 and follow-up extended for 365 days or until death, whichever happened earlier. If the patient did not use any healthcare resources during the covered data collection period, the follow-up time was assumed to be the calendar year 2011.
Prevalence of AF in Joensuu was approximated on the basis of the number of patients with AF in the study centre and the number of inhabitants17 in Joensuu in the following age groups: <50, 50–59, 60–69, 70–79 and 80 years or older. Warfarin treatment quality was assessed as the proportion of time spent in the therapeutic INR range (TTR). TTR was estimated with the Rosendaal method.18 Patients were considered to be warfarin users during the follow-up period if their TTR was analysable from the database (ie, the patients had at least two separate INR test measurements during the defined 1-year follow-up period or one test during the follow-up period and at least one INR test measurement immediately prior to or after the defined follow-up period). As most, but not all, of the patients had individually specified INR ranges in the database, TTR was estimated (referred to as ‘individual TTR’ below) by using the individual INR target range when it was known and an assumed 2.0–3.0 target range, when individual targets had not been specified. Also TTR based on an assumed 2.0–3.0 interval for all warfarin users was estimated (referred to as ‘standard TTR’ below). The analyses were repeated for all warfarin users and for the subgroup of patients using warfarin continuously. Continuous warfarin use was defined as warfarin use without gaps exceeding 56 days between two successive INR measurements. This definition is based on the Finnish guidelines on implementing warfarin treatment in which a threshold of 4–8 weeks is mentioned as a usual maximum gap between successive INR.19 The proportion of patients with the following TTR ranges was additionally analysed: TTR<40%, 40%≤TTR<60%, 60≤TTR<70%, 70%≤TTR<80% and TTR≥80%. The classification was based on the finding that TTR exceeding 70% is associated with significantly longer time to stroke, whereas TTRs below 40% provide no survival advantage compared with no warfarin treatment.20
The healthcare costs for the patient cohort were estimated by multiplying the use of healthcare resources with the unit costs of those resources. For comparative purposes, the unit costs (variable and fixed costs included) were obtained from two different sources: the national Finnish healthcare unit costs21 and unit costs of the service provider. The national unit costs were converted to year 2011 values using the latest official communal healthcare price index,22 that is, the conversion factor from year 2006 was 1.171. The applied national and service provider unit costs were €71.30 and €119.95 for a physician visit, €20.00 and €48.48 for a physician phone consultation, €31.50 and €56.31 for a nurse visit, €8.84 and €18.77 for a nurse phone consultation, €165.35 and €153.00 for an inpatient day and €7.84 and €13.80 for an INR test, respectively. Since the cost of a nurse phone consultation was not reported in the national unit cost report, we applied the relative difference between the cost of a physician and a nurse visit to the cost of a physician phone consultation to obtain the cost of a nurse phone consultation (31.50/71.30×20.00=€8.84). Similarly, we estimated the costs for nurse and physician phone consultations in Joensuu by applying the relative difference between the cost of a visit and the Joensuu list price for a visit to the list price of the respective phone consultations (ie, cost for physician phone consultation: €119.95/€146.80×€59.33=€48.48; cost for nurse phone consultation: €56.31/€73.95×€24.65=€18.77). The national unit costs for primary healthcare inpatient days vary from €165.35 in primary healthcare wards to €369.84 in specialist-led primary healthcare hospitals. In order to avoid overestimating the costs, we used the cost estimate for primary healthcare wards (ie, the lowest cost), since we had information only on the diagnosis codes and the number of inpatient days but not the intensity or specific reason for the treatment obtained.
To explain the individual variations in treatment costs, a generalised linear regression model (GLM, with γ distribution and logarithmic link function, see eg, Griswold et al23) was estimated using the following explanatory variables: age, gender (1=men, 0=women), number of inpatient days, length of follow-up in days, comorbidities (as dummy variables with 1=yes, 0=no), use of home nursing services (1=yes, 0=no) and a categorical variable classifying patients into the above mentioned TTR range groups and non-users of warfarin. The dummy variables and categorical variables were handled as factor variables in STATA MP V.11.2 to allow correct estimation of marginal effects of the analysed covariates (ie, the change in treatment costs as the covariate changes). Marginal independent effects for each variable were predicted at sample mean values for other covariates except for the duration of follow-up, which was fixed at 365 days. The costs of home nursing services and primary healthcare inpatient stays were excluded from the analyses because the information regarding the specific reasons for inpatient days/home nursing service use and the intensity (ie, number of visits during the follow-up period) of home nursing service use were not collected/known. We assume that warfarin use is not the primary reason for needing home nursing services or primary healthcare inpatient stays in Finland. Explanatory variables with p≤0.05 were considered to have a significant impact on the outcome variable.
No ethical approval or informed patient consent was required for the study according to Finnish laws because patients were not contacted and only anonymous patient data were used in the analyses.
Results
Patient characteristics and prevalence of AF
The patient cohort consisted of 2746 patients with AF. In total, 52.8% of the patients were men and the average age of the patients was 74.3 years (SD: 12.3). The average CHA2DS2-VASc and CHADS2 scores were 3.1 (SD: 1.7, median 3, IQR 3) and 1.6 (SD: 1.4, median 1, IQR 2), respectively.
The average prevalence of AF in Joensuu was 3.7%, being 4.1% for men and 3.4% for women. Based on the age and gender structure of the population in Joensuu, the prevalence of AF was estimated to be 0.36% and 0.06% in men and women aged <50 years, 3.6% and 0.9% in men and women aged 50–59 years, 8.3% and 3.4% in men and women aged 60–69 years, 20.6% and 11.3% in men and women aged 70–79 years and 34.9% and 30.7% in men and women aged 80 years or older, respectively.
In June 2012, the following proportions of patients were prescribed antithrombotic medications according to the data in the primary care database: 51.8% warfarin, 15.5% acetylsalicylic acid, 1.2% clopidogrel, 0.6% dabigatran and 0.1% rivaroxaban. When warfarin use was assessed based on analysable TTR during the follow-up period, the proportion of all patients using warfarin dropped to 46.3% (among patients with CHA2DS2-VASc≥1, 50.3% of patients used warfarin). However, at least one INR test had been performed for altogether 71.4% of the patient cohort before, during or after the follow-up period.
The patients using warfarin were significantly older and had more comorbidities than the patients not using warfarin. The detailed patient characteristics are shown in table 1.
Patient characteristics
Quality of warfarin treatment
The INR target ranges were individually defined for 1161 (91.3%) of 1271 patients who used warfarin during the observation period. The individually defined INR targets were the following: 1.5–2.5 (n=1), 1.7–2.5 (n=1), 2.0–2.5 (n=7), 2.0–3.0 (n=1078), 2.5–3.5 (n=71), 2.5–4.0 (n=1) and 3.0–3.5 (n=2). The vast majority of patients (92.9%) with a specified target range, therefore, aimed at the recommended 2.0–3.0 INR range for chronic AF, and the specified target range (2.5–3.5) used in 6.1% of the patients matches the recommendation for patients with mechanical heart valves.19
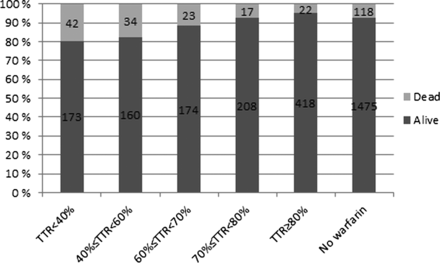
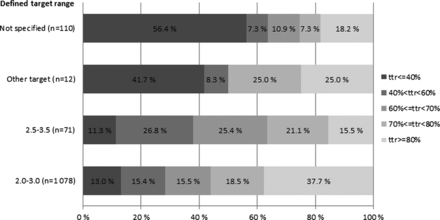
The average TTRs based on individual targets and standard target range (ie, 2.0–3.0) among all warfarin users were 65.2% (SD: 26.8) and 64.4% (SD: 26.9), respectively (see table 2). The proportion of patients with TTR<40%, 40%≤TTR<60%, 60≤TTR<70%, 70%≤TTR<80% and TTR≥80% were 16.9%, 15.3%, 15.5%, 17.7% and 34.6%, respectively. More patients had died in the subgroups with poorer treatment balance by the time of data collection (figure 1). The proportion of patients with poor treatment balance (TTR<40%) was higher among patients without individually specified target INR ranges in the database (56.4%) and patients with ‘atypical’ INR target ranges (41.7%) as compared with patients with a target range of 2.0–3.0 (13%) or 2.5–3.5 (11.3%; figure 2).
Average time in TTR among warfarin users
Number of patients alive and dead (in June 2012) in each TTR subgroup. TTR, time in therapeutic range.
Proportion of patients reaching different TTR levels according to patients’ defined target INR range. Defined target range, individual INR target range specified in the database. TTR, time in therapeutic range.
Healthcare resource use and costs
The average annual outpatient costs in the patient cohort were €314.44 (95% CI €298.74 to €330.13) with national unit costs and €560.26 (95% CI €532.40 to €588.12) with service provider costs. The use of healthcare resources and associated costs are reported in table 3.
Healthcare resource use and average costs (2011 value)
The patients using warfarin had, on average, 19.1 INR tests (SD: 13.7, median 17.0) during the follow-up period (average time between first and last INR test result was 336.3 days, SD: 78.3). The average number of tests was 11.1 (SD: 12.9, median 6.0) for patients with individual TTRs below 40% (average time between first and last INR test result was 280.4 days, SD: 123.8), 30.7 (SD: 18.5, median 28.5) for patients with individual TTRs between 40% and 60% (average time between the first and the last INR test result was 334.6 days, SD: 75.3), 23.5 (SD: 12.2, median 22.0) for patients with individual TTRs between 60% and 70% (average time between the first and the last INR test result was 347.2 days, SD: 60.0), 21.7 (SD 11.1, median 20.0) for patients with individual TTRs between 70% and 80% (average time between the first and the last INR test result was 353.4 days; SD: 44.5) and 14.6 (SD: 7.8, median 14.0) for patients with individual TTRs equal or above 80% (average time between the first and the last INR test result was 350.6 days; SD: 56.8).
The results of the performed GLM regression analyses (excluding costs of inpatient stays and home nurse visits) are depicted in table 4. Diagnosis of angina pectoris, diabetes and neoplasms increased treatment costs significantly, whereas male gender was associated with slightly lower costs compared with female gender. When Finnish national unit costs were applied, the costs were €48.53 lower for male patients compared with female patients. Similarly, angina pectoris, diabetes and neoplasms increased the costs by €61.27, €54.70 and €269.58, respectively. The annual costs of warfarin users were, on average, €524.11 (95% CI €449.71 to €598.52, p<0.001) higher than the costs of non-users. The increase in cost varied from €417.90 to €689.86, depending on the quality of warfarin treatment that was measured with the TTR class. When the analyses were performed with service provider costs, the average annual costs were €939.54 (95% CI €806.81 to €1072.27, p<0.001) higher among warfarin users and the cost increase varied from €752.55 to €1233.90 depending on the patients’ TTR class (results of the analysis are presented in online supplement 1).
Factors affecting total treatment costs (using the national unit costs) and marginal effect of covariates (n=2738)
Discussion
In the study centre, warfarin treatment was used by approximately 50% of such patients with AF for whom the Finnish treatment guidelines recommend oral anticoagulation treatment (ie, CHADS-VASc≥1). The warfarin users were in the target INR range for approximately 65% of the time. The TTR was, on average, 10 percentage points greater among patients using warfarin continuously during the follow-up period. The findings suggest that, on an average, the quality of treatment with warfarin was good in the study centre. However, one-third of patients had difficulties maintaining their target INR ranges, which was reflected in a TTR of below 60%. The outpatient costs were higher among warfarin users: the increase in cost varied from €417.90 to €1233.90, depending on the achieved TTR and used unit cost estimates for healthcare resources.
Some studies have assessed the quality of treatment with warfarin in a real-life setting.11 ,24–27 TTR or alternatively the proportion of INR tests within target range in these studies varied from 57.8% to 76.2%. Corresponding estimates from Finnish studies ranges from 63.4% to 66.4%.11 ,27 ,28 The key strength of our study is the estimation of TTR on the basis of the INR ranges that have been defined for the individual patients based on their needs and clinical rationale. In contrast, most published studies simply assume that the target range is 2.0–3.0. For comparative purposes, we also reported the results based on assumed 2.0–3.0 INR target range. Despite differences between individually defined and assumed TTRs in our study, our study findings are well in line with previous Finnish studies, suggesting that the results of this study are likely to be generalisable also to other Finnish service providers.
The study cohort consisted of almost equal number of men and women even though age-specific prevalence of AF was higher in men. This observation is explained by the unequal gender distribution in Joensuu municipality: 62.2% of all inhabitants aged 70 years or older were women in 2011.17 Compared with other estimates on the prevalence of AF2 ,29 that have been published, the prevalence of AF was observed to be clearly higher in our study centre. The figures for each age group are very similar to those reported for a cohort in the USA and the Netherlands that was carried out a decade earlier.2 ,29 This observed difference may relate to the fact that the overall incidence of cardiovascular diseases in Finland is higher than, for example, in South-European and West-European countries.30 ,31
Another interesting finding of our study was that the patient characteristics and TTRs among warfarin users were very similar to those in the ARISTOTLE5 and RELY4 trials. This could mean that the generalisability and applicability of those trial results are good for Finland. For example, the mean TTR among the Finnish patients participating in the RELY-trial was 74%.32 A close match was also observed in the CHA2DS2 scores, age and most comorbidities with the exception of hypertension. In a representative sample of Finnish citizens, 54% and 43% of men and women, respectively, had increased blood pressure (≥140/90 mm Hg and/or treatment for hypertension).33 Therefore, we suspect that hypertension is an underused diagnosis code in the medical database and that the proportion of patients with increased blood pressure in Joensuu is, in reality, far greater than the reported 14%.
At the time of data collection, a larger proportion of patients with low TTR during the follow-up period had died when compared with patients with higher TTR or no warfarin use. Although this observation was interesting, we could not assess whether the deteriorating condition of patients prior to their death leads to lower TTR or whether the lower TTR leads to higher risk of death. This would be an interesting subject for further study.
Our approach of using numerical data directly from the local primary care databases has some limitations. The accuracy of the numerical data compared with written medical reports was not checked and the causal relationships between warfarin use and use of healthcare resources were not formally assessed. Therefore, we cannot conclude that the observed cost differences were due to warfarin use alone. For instance, the costs for warfarin users and non-users might also have differed as a result of the severity of comorbid conditions. However, our data included only the diagnoses, but not the severity of the disease or time from diagnosis. Similarly, the reasons for individually defined INR target ranges deviating from those recommended for patients with chronic AF or mechanical heart valves are not known but we assume the deviations to be related to individual tailoring based on the patient's previous experiences with warfarin treatment. It should also be emphasised that secondary healthcare costs associated with AF complications were beyond the scope of our study, and therefore we did not assess any potential cost differences that might result from the reduced risk of those complications.
Institutional data are also prone to human error and omissions. Some diagnosis codes may have been under-reported and a large number of diagnosis codes were missing for some health services, especially phone consultations. Nevertheless, the annual cost of warfarin treatment has been previously estimated as approximately €590 for users in Finland using the national unit costs,11 and the results of our study on the national unit costs are well in line with this estimate. An additional limitation was the exclusion of all drug costs in the assessment. We chose not to include these costs for two reasons: we lacked the information on drug doses and we could not reliably confirm the duration of use for drugs other than warfarin. Warfarin use was confirmed through regular INR testing. However, we lacked information on whether the patients actually acquired warfarin from pharmacies and how adherent they were to treatment. In addition, new anticoagulants were not handled separately in the analysis since only a few patients used them during the study period. Rivaroxaban and dabigatran were granted marketing authorisation for the prevention of stroke and systemic embolism in patients with AF during the study period but neither was reimbursable for this indication by the Social Insurance Institution of Finland.
We estimated TTR using three different definitions. The least restrictive definition included all patients with two INR measurements regardless of the time interval between these measurements. From clinical perspective, this definition may be too broad, and therefore TTR was also estimated for patients with maximum gap of 8 weeks between two successive measurements (ie, continuous warfarin use), and patients with continuous warfarin use for the entire follow-up period. The 8-week maximum gap was chosen to overcome the lack of information regarding temporary treatment discontinuations. The lowest TTRs were observed with the least restrictive TTR definition and vice versa. The estimated TTRs for continuous warfarin use during the follow-up period were approximately 8–9% higher when compared with the least restrictive definition.
The primary care costs in Joensuu differ from those reported in the Finnish national unit cost report. The national unit costs are, for some parts (such as the cost of primary healthcare services), based on the relatively old cost estimates of only one or a few service providers and may not be representative for the whole country. When we estimated total costs using national unit costs, the total cost estimate was just over 50% of the estimate obtained when service provider costs were used. Considering the fact that the primary healthcare outpatient cost per inhabitant in the studied municipality are among the lowest of similar size Finnish municipalities,34 the cost estimates presented in this study may slightly underestimate the average Finnish cost for patients with AF. Furthermore, our study suggests that, when the cost-effectiveness of new treatments is assessed based on the national unit costs, the overall cost-effectiveness of new treatments that reduce the use of healthcare resources may actually be underestimated. This finding underlines the importance of obtaining accurate cost-information to support rational healthcare resource allocation.
Conclusions
In our real-life study reflecting contemporary treatment practice, approximately 50% of patients with AF were being treated with warfarin. The average TTR among patients using warfarin was 65.2%, increasing to 74.5% among the patients using warfarin during the entire follow-up period. The obtained high TTRs suggest that the patients using warfarin had, on average, good INR balance, yet one-third of patients had TTR below 60%. The patient characteristics and attained INR balance were very similar to those in recent clinical trials assessing the efficacy of new anticoagulants. The primary care outpatient costs were higher among warfarin users and the costs had an inverse relationship with achieved TTR.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online supplement
Footnotes
-
Contributors TH, CA and EJS contributed to study design. EJS, AL and TH contributed to study management. TH, AL and EJS contributed to data collection. CA and TH contributed to data assembly for statistical analysis. TH and EJS designed statistical analyses which TH carried out. TH drafted the first version of the manuscript. All authors contributed to the interpretation of the results and provided input on drafts of this article. All authors approved the final version of the manuscript.
-
Funding This study was supported and funded by Pfizer Oy, Helsinki, Finland and Oy Bristol-Myers Squibb (Finland) Ab (BMS), Espoo, Finland. The sponsors reviewed the manuscript and influenced the choice of journal.
-
Competing interests TH, EJS and CA are employees and shareholders of ESiOR Oy which was commissioned by BMS/Pfizer to perform this study. EJS is the CEO of ESiOR Oy. AL and PK has received financial support from Pfizer to cover travel costs to an educational seminar.
-
Provenance and peer review Not commissioned; externally peer reviewed.
-
Data sharing statement No additional data are available.