Article Text
Abstract
Objectives To evaluate the relationship between the use of non-aristolochic acid (AA) prescribed Chinese herbal medicines (CHMs) and the risk of mortality in patients with chronic kidney disease (CKD).
Design Nationwide population-based follow-up study.
Setting Longitudinal health insurance database sampled from the Taiwan National Health Insurance Research Database.
Participants A total of 47 876 patients with CKD were identified. Participants who had ever used AA-containing CHMs, had cancer or HIV prior to the diagnosis of CKD, died within the first month of CKD diagnosis and who were not Taiwanese citizens were excluded. A total of 13 864 participants were eligible for final analysis.
Primary and secondary outcome measures All-cause mortality among patients with CKD between 2000 and 2008.
Results After controlling for potential confounders, we found that participants who started to receive non-AA prescribed CHMs after the diagnosis of CKD had a lower risk of mortality as compared with non-users of non-AA prescribed CHMs (adjusted HR (aHR) 0.6; 95% CI 0.4 to 0.7, p<0.001). Moreover, participants who had used non-AA prescribed CHMs prior to and after the diagnosis of CKD also had a lower risk of mortality than non-users (aHR 0.6; 95% CI 0.5 to 0.8, p<0.001). In subgroup analyses, we found that such an inverse association was present only among patients who were not eligible to receive erythropoietin therapy (ie, serum creatinine ≦6 mg/dL and/or haematocrit value ≧28%).
Conclusions Patients who received non-AA prescribed CHMs after the diagnosis of CKD, yet before the start of erythropoietin therapy had a lower risk of mortality than those who did not.
- Chronic Kidney Disease
- Prescribed Chinese Herbal Medicine
- Aristolochic Acid
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/
Statistics from Altmetric.com
Strengths and limitations of this study
-
This is the first large-scale population-based follow-up study to evaluate the relationship between the use of non-aristolochic acid (AA) prescribed Chinese herbal medicines (CHMs) after the diagnosis of chronic kidney disease (CKD) and the risk of mortality in patients with CKD.
-
The major limitations of the study were similar to those inherent of a retrospective follow-up study.
-
Other study limitations included the difficulty in identifying specific ingredients of non-AA prescribed CHMs that might be beneficial to patients with CKD, the lack of information on non-prescribed CHMs and renal function indices, and the unavailability of lifestyle factors that could potentially confound the relationship between the use of non-AA prescribed CHMs and the risk of mortality.
Introduction
Chronic kidney disease (CKD) is a common chronic disease with an estimated global prevalence of approximately 8–16%.1 CKD is also one of the main causes that contribute to the increasing mortality rate around the world.2 Taiwan has the highest incidence of CKD and end-stage renal disease (ESRD) worldwide3 and the use of Chinese herbal medicines (CHMs) is very popular in Taiwan. Previous studies had found that CHMs containing aristolochic acid (AA), a carcinogenic substance to human beings, were correlated with acute renal failure4 and urinary tract carcinoma.5 A large population-based study had also shown that the intake of any kinds of CHM was associated with the occurrence of CKD in Taiwan.6 However, in a recently published case–control study, the occurrence of CKD was found to be limited to participants who received non-prescribed CHMs, but not those who consumed prescribed CHMs.7 Moreover, some commonly prescribed CHMs such as Astragalus membranaceus (huang qi), Angelica sinensis (dong quai) and Rheum palmatum (da huang, rhubarb root) were suggested to possess renoprotective effects.8 Given that the association between the use of prescribed CHMs and the occurrence and/or disease progression of CKD remains unclear, we conducted a nationwide population-based follow-up study to better understand the use of non-AA prescribed CHMs after the diagnosis of CKD and the effect of such medication use on the risk of mortality in patients with CKD.
Materials and methods
Study population
This retrospective follow-up study employed data derived from the National Health Insurance Research Database (NHIRD) in Taiwan to identify the study population. The NHIRD was established in 1996 and became more comprehensive in terms of data compilation in 1997. This database contains more than 96% of the entire Taiwanese population’s medical information, including outpatient and emergency department visits, hospitalisation, medication use (conventional medicines and CHMs) and diagnostic data (coded by International Classification of Diseases, Ninth Revision, Health Problems, ICD-9-CM).9 The database used for this study is the longitudinal health insurance database randomly sampled by the Taiwan NHI Bureau in 2000 (Longitudinal Health Insurance Database 2000; LHID2000), which consists of one million Taiwanese population who were insured in that year. The LHID2000 contained follow-up medical information, spanning from 1997 to 2008, for the aforementioned cohort. The sampling method of the LHID2000 was completely random; and the distribution of age, gender and other characteristics of the sampled cohort was validated by the Taiwan National Health Research Institutes. Moreover, hundreds of studies utilising this database had been published in peer-reviewed journals and the database was believed to be comprehensive and reliable.10 ,11
Eligible participants and duration of follow-up
Within the LHID2000, we identified patients with CKD by searching for the following ICD-9-CM codes: 581–583, 585–589, 250.4, 274.1, 403 and 404. When a patient had at least three consecutive outpatient visits (equivalent to approximately 3 months of follow-up) or a hospitalisation record for the aforementioned ICD-9-CM codes, they were defined as a patient with CKD. We selected the aforementioned ICD codes after consulting a group of nephrologists to better understand the practice of coding CKD diagnosis in the NHIRD and taking into account the definition of CKD in previously published papers that also employed the NHIRD.12–14 To ensure that there was no pre-existing CKD and there was sufficient time (at least 3 years) to assess the participants’ comorbidities and medication use prior to the diagnosis of CKD, we included only participants who had a first-time diagnosis of CKD on or after 1 January 2000. Moreover, all participants must be diagnosed with CKD prior to 31 December 2007 to ensure a follow-up time of at least 1 year.
Because AA-containing CHMs were proven to cause acute renal toxicity and urothelial cancer,4–6 we excluded participants who had taken any of the following AA-containing medications before the diagnosis of CKD: Ma-Dou-Ling (Fructus aristolochiae), Tian-Xian-Teng (Caulis aristolochiae), Xi-Xin (Asarum heterotoppoides), Guan-Mu-Tong (Aristolochia manshuriensis), Guang-Fangchi (Aristolochia fangchi), Qing-Mu-Xiang (Radix aristolochiae), Mu-Tong (Akebia spp), Fangchi (Stephania spp) and Mu-Xiang (Radix aucklandiae).5 ,15 Participants were also excluded if they were diagnosed with cancer prior to the diagnosis of CKD, had HIV infection and were not Taiwanese citizens. Finally, because patients who died within 1 month after the diagnosis of CKD were found to be less likely to receive non-AA prescribed CHMs (data now shown) probably because they had a more severe underlying disease or were even in a moribund state, we excluded such participants to minimise the possibility of selection bias.
All eligible participants were then followed from the time of incident diagnosis of CKD until death or 31 December 2008, whichever came first. The outcome of interest was the risk of all-cause mortality in patients with CKD during the follow-up period. Death was defined as withdrawal of the patients with CKD from the NHI programme.
Assessment of CHM use
All CHMs prescribed by certified Chinese medicine practitioners and manufactured following ‘Good Manufacturing Practice’ are eligible to be reimbursed by the NHI Bureau in Taiwan and one cannot purchase prescribed CHMs in Taiwan without a certified Chinese medicine practitioner’s prescription. Therefore, the LHID2000 contained all information on prescribed CHMs, and we were able to assess the participants’ overall use of prescribed CHMs during the study period, including the names and the amount of prescribed CHMs.16 Moreover, according to the standard prescriptions recommended by the Committee of Chinese Medicine and Pharmacy (CCMP) in Taiwan, the ingredients of each compound CHM could also be identified.17 After the incident diagnosis of CKD, each participant's use of non-AA prescribed CHMs, including the date, amount and duration of each prescription was assessed. Owing to the complexity of prescribed CHMs, which could comprise either a single ingredient or a compound product (ie, a mixture of different CHM ingredients) and the prescribed medications could be manufactured in powder, liquid, tablet or other forms, the cumulative exposure dose of individual CHMs was extremely difficult to determine. Therefore, we eventually divided the use of non-AA prescribed CHMs into non-use, use only prior to the diagnosis of CKD, use only after the diagnosis of CKD and use in both periods before and after the diagnosis of CKD.
Assessment of potential confounders
To control for the effects of potential confounders, we obtained the data on age, gender, comorbidities, prior hospitalisation, concomitant use of non-steroidal anti-inflammatory drugs (NSAIDs) and other analgesics (including acetaminophen, buprenorphine, codeine, meperidine, morphine, nalbuphine and tramadol that were reimbursed by the Taiwan NHI Bureau during the study period), and the receipt of erythropoietin therapy within the LHID2000 database. We classified age into three groups, that is, less than or equal to 40, 40–65 and greater than 65 years. For pre-existing comorbidities, we paid special attention to hypertension, diabetes mellitus and cardiovascular disease (CVD) (including congenital heart disease) and ascertained relevant diagnoses by searching for three consecutive outpatient visits and/or any hospitalisation for the ICD-9 codes of individual diseases within 1 year before the incident diagnosis of CKD. We also employed Charlson’s Comorbidity Index (CCI)18 to control for the overall disease burden of various comorbidities on each study participant. The definitions of comorbidities other than hypertension, diabetes mellitus and CVD were similar to those of the aforementioned diseases. We further defined participants who were hospitalised within 1 year prior to the diagnosis of CKD as having any ‘hospitalization before the diagnosis of CKD’.
In addition to comorbidities, we assessed the participants’ overall medication history, including the date, amount and duration of prescriptions before and after the diagnosis of CKD.16 We specifically examined the use of NSAIDs, analgesics and erythropoietin, which were likely to confound the relationship between the use of non-AA prescribed CHMs and the risk of mortality in patients with CKD. We divided the use of NSAIDs and analgesics by the annual total amount of pills received by the study participants within the previous year into ≦104 and >104 pills.19 The receipt of erythropoietin, a surrogate marker of the severity of CKD in Taiwan because its use was strictly limited to patients with ESRD who had a haematocrit (Hct) value less than 28% and/or patients with CKD who had a serum creatinine greater than 6 mg/dL and a Hct value less than 28%, was classified as use or non-use.
Statistical analyses
We employed log-rank test and Holm-Sidak multiple comparisons to examine the survival rate between patients with different patterns of non-AA prescribed CHM use before and after the diagnosis of CKD. We further performed Cox proportional hazard regression to assess the association between various predictors and study participants’ survival rates. We also conducted subgroup analyses by stratifying on the surrogate marker of the severity of CKD, that is, the receipt of erythropoietin therapy, to see whether the severity of CKD would modify the effect between non-AA prescribed CHMs and the risk of mortality. Moreover, we performed sensitivity analysis by excluding participants whose CKD was defined by ICD codes other than 585 (chronic renal failure) and 586 (renal failure, unspecified). All variables with a p value less than 0.05 in univariate analyses were entered into the multivariate analyses. All statistical analyses were performed with the SAS statistical package V.9.1.3 (SAS Institute, Inc, Cary, North Carolina, USA).
Results
A total of 47 876 participants had an incident diagnosis of CKD after 1 January 2000. Among them, 32 000 had ever used AA-containing prescribed CHMs and were thus excluded. We further excluded 60 participants who were not Taiwanese citizens and 1882 participants with any type of cancer and/or HIV infection before the diagnosis of CKD. Seventy participants who died within the first month of CKD diagnosis were excluded as well. This resulted in a total of 13 864 participants eligible for final analysis.
The study participants had a mean age of 54.2±20.9 years. A total of 929 participants (6.7%) died during the follow-up period between 2000 and 2008. In univariate analyses, the mortality rate was higher in men as compared with women. Older participants also had a higher mortality rate than the younger ones. Moreover, participants who had a higher CCI score, were hospitalised within 1 year prior to the diagnosis of CKD and were diagnosed with hypertension, diabetes mellitus and CVD, were found to have a higher mortality rate. The receipt of greater than 104 pills of NSAIDs and/or analgesics annually, and the treatment with erythropoietin were associated with a high risk of mortality as well.
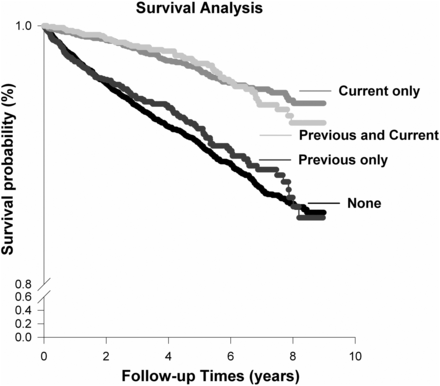
Use of non-AA prescribed CHMs was fairly common among patients with CKD with 25.1% of the survivors and 14.4% of the deceased received non-AA prescribed CHMs after the diagnosis of CKD (table 1). The crude mortality rate appeared to be highest among those who never received non-AA prescribed CHMs (ie, non-use), followed by those who received non-AA prescribed CHMs only prior to the diagnosis of CKD, those with non-AA prescribed CHMs use only after CKD, and those with prescribed CHMs use before and after the diagnosis of CKD. The observed trend between different groups of non-AA prescribed CHMs use was statistically significant (table 1). The study findings further indicated that receipt of non-AA prescribed CHMs after the diagnosis of CKD, with or without the use of non-AA prescribed CHMs before CKD diagnosis, had a significantly better survival rate than those who never received or those who received non-AA prescribed CHMs only before the diagnosis of CKD (p<0.001; figure 1).
Distribution of baseline characteristics of 13 864 patients with CKD in Taiwan
Survival curve of 13 864 patients with chronic kidney disease (CKD), defined by their use of non-aristolochic acid (AA) prescribed Chinese herbal medicines (CHMs) before and after the diagnosis of CKD note: group A: non-use of non-AA prescribed CHMs; group B: use of non-AA prescribed CHMs only before the diagnosis of CKD; group C: use of non-AA prescribed CHMs only after the diagnosis of CKD; group D: use of non-AA prescribed CHMs before and after the diagnosis of CKD. Log-rank test (Holm-Sidak method): group A versus C, p<0.001; group A versus D, p<0.001; group B versus C, p<0.001; group B versus D, p<0.001.
In the multivariate analysis that employed the non-use group as the reference, we found that the mortality rate was similar among those with non-AA prescribed CHMs use only before the diagnosis of CKD. By contrast, those participants who received non-AA prescribed CHMs only after the diagnosis of CKD had a lower risk of mortality in the follow-up period (adjusted HR (aHR) 0.6; 95% CI 0.4 to 0.7; p<0.001). Moreover, participants with non-AA prescribed CHMs use before and after the diagnosis of CKD also showed a lower mortality rate (aHR 0.6; 95% CI 0.5 to 0.8; p<0.001; table 2).
Cox proportional hazard analysis of the predictors of all-cause mortality in 13 864 patients with CKD in Taiwan
In the subgroup analyses stratified by the receipt of erythropoietin therapy, we found that the inverse association between the use of non-AA prescribed CHMs and the risk of mortality was present only among patients who did not receive erythropoietin therapy (aHR 0.3 (95% CI 0.3 to 0.4) for patients who received non-AA prescribed CHMs only after the diagnosis of CKD; and aHR 0.4 (95% CI 0.3 to 0.5) for patients who received non-AA prescribed CHMs before and after the diagnosis of CKD). It is also noteworthy that the use of non-AA prescribed CHMs was not associated with a higher risk of mortality among patients who received erythropoietin therapy (data not shown).
In the sensitivity analysis that included only participants whose CKD diagnosis was defined by ICD codes 585 and/or 586, the number of study participants decreased from 13 864 to 7159; however, the inverse association between non-AA prescribed CHMs and all-cause mortality among patients with CKD remained statistically significant. For patients who received non-AA prescribed CHMs only before the diagnosis of CKD, the aHR was 0.9 (95% CI 0.7 to 1.1); whereas the aHR was 0.6 (95% CI 0.5 to 0.8) and 0.5 (95% CI 0.3 to 0.7) for patients who received non-AA prescribed CHMs only after the diagnosis of CKD and for patients who received non-AA prescribed CHMs before and after CKD, respectively.
Discussion
Compared with participants without CKD, the occurrence of cardiovascular events and the risk of mortality were higher among patients with CKD.20 Therefore, identifying possible treatments that can delay the progression of CKD is important in the prevention of mortality among patients with CKD. Although the use of CHMs was frequently associated with the risk of CKD, the exact relationship between the use of CHMs and the occurrence and/or progression of CKD remains unclear. In this study, we employed a nationwide population-based database to investigate the effects of non-AA prescribed CHMs on the risk of mortality in patients with CKD. We found that among patients with CKD, men, older age, the presence of certain comorbidities (ie, hypertension, diabetes mellitus and CVD), receipt of higher dose of analgesics, treatment with erythropoietin and hospitalisation prior to the diagnosis of CKD were associated with a higher risk of mortality; whereas the use of non-AA prescribed CHMs after the diagnosis of CKD yet before the start of erythropoietin therapy was related to a lower risk of mortality.
Many studies have investigated the association between CHMs use and the risk of CKD and/or ESRD, and there was plenty of evidence suggesting a likely causal relationship between the consumption of AA-containing CHMs and the development of acute renal failure,4 CKD, ESRD13 and urothelial cancer.5 Studies that did not differentiate between AA-containing CHMs and non-AA CHMs thus might overestimate the adverse effects of non-AA CHMs. For example, Wen et al6 found a positive association between CHM use and the risk of CKD. However, it was unclear whether the observed association was attributable to AA-containing CHMs or other CHMs. The inability to differentiate between AA-containing CHMs and non-AA CHMs had also resulted in the prejudicial term ‘Chinese herbal nephropathy’ and led many western medicine practitioners to warn the patients with CKD against the use of any CHMs. In this study, we noticed that nearly two-thirds of those patients who had used prescribed CHMs prior to the diagnosis of CKD discontinued the treatment after the diagnosis of CKD. However, not all CHMs were harmful. In fact, some animal experiments and clinical studies have shown that the use of certain CHMs could protect against the development of CKD or delay the deterioration of CKD.8 ,21–24 In our previous study, we also found that CKD/ESRD was related to the use of non-prescribed CHMs, but not non-AA prescribed CHMs.7 The aforementioned findings suggested that non-AA CHMs, appropriately prescribed by certified Chinese medicine practitioners, could have a renoprotective effect or at least a non-nephrotoxic effect in patients with CKD.
In this study, we excluded all participants who had used AA-containing CHMs before the diagnosis of CKD to eliminate the likely strong confounding effects of AA. Our study results showed that patients with CKD who received non-AA prescribed CHMs after the diagnosis of CKD, with or without the use of non-AA prescribed CHMs prior to CKD, had a significantly lower risk of mortality than non-users or those who discontinued non-AA prescribed CHMs after the diagnosis of CKD. In the subgroup analyses stratified by the receipt of erythropoietin therapy, we further found that the above-noted inverse association was present only among patients whose CKD was not severe enough to warrant erythropoietin therapy. Given that the function of kidney exponentially declines as CKD progresses, the use of and the response to non-AA prescribed CHMs might be significantly different between patients with different stages of CKD. The findings of this study thus supported the proposition that non-AA CHMs prescribed by certified Chinese medicine practitioners might be helpful in delaying the progression of CKD and/or its complications (eg, CVD) in patients with earlier stages of CKD and subsequently reducing their risk of mortality.
In Taiwan, according to the unified formulas of CHMs provided by the Taiwanese CCMP, the majority of prescribed CHMs are compound (cocktail) formulas.17 The CHM formulas often contain ‘renoprotective’ CHM ingredients such as Astragalus membranaceus, Angelica sinensis and Rheum palmatum. These CHMs were found to inhibit renal tubulointerstitial fibrosis,24 enhance nitric oxide production and alleviate renal interstitial fibrosis,23 and lower serum cholesterol, blood urea nitrogen, creatinine, oxidative stress and profibrotic factors to attenuate nephritis and renal necrosis.8 Although there was no existing large-scale randomised controlled trials to support the beneficial effects of the aforementioned CHMs, our findings did indicate that the use of such non-AA prescribed CHMs might reduce the mortality risk in patients with CKD. Further studies are warranted to delineate the exact ingredients and mechanisms of non-AA prescribed CHMs that can delay the progression of CKD and/or its complications.
In addition to non-AA prescribed CHMs, we also found that male gender and older age were associated with a higher risk of mortality in patients with CKD, a finding consistent with previous reports.19 ,20 Female sex hormones are known to inhibit inflammatory and apoptotic processes, which can then protect renal tissues.25 Therefore, female gender is considered as a protective factor in many kidney diseases and a lower mortality rate caused by CKD has been observed in women as compared with men. By contrast, older age and increased comorbidities have been associated with higher disease severity and mortality rate.26
In this study, several comorbidities were associated the risk of mortality in patients with CKD as well. Among them, hypertension is a well-known risk factor for the progression of CKD and abnormal blood pressure would cause the deterioration of CKD, which can lead to cardiovascular complications and mortality.27 Diabetes mellitus is also an important risk factor for the progression of CKD. According to previous reports, abnormal glycated haemoglobin can result in a higher probability of CVD as well as macroalbuminuria. These complications then lead to faster progression of CKD, or even death.28 Many literature reports had further indicated that CVD is the major complication as well as the cause of mortality in patients with CKD.29 Therefore, the observed association between hypertension, diabetes mellitus and CVD and a higher risk of mortality in patients with CKD seems plausible.
The risk of mortality was also related to the use of analgesics and erythropoietin and any hospitalisation prior to CKD diagnosis. The use of analgesics was believed to be related to CKD deterioration,19 and our finding on the use of analgesics did suggest such an association. Erythropoietin is a type of glycoprotein hormone, which is used in the regulation of haemoglobin. The use of erythropoietin therefore indicates a deficiency in the production of renal erythropoietin and suggests the need of dialysis or worst renal function in the recipients. Similarly, if a participant was hospitalised prior to the diagnosis of CKD, they were likely to be in a poor health condition. Both variables were therefore expected to be associated with a higher risk of mortality among patients with CKD, as evidenced in this study.
Some previous studies had shown that the use of NSAIDs was related to the deterioration and subsequent mortality in patients with CKD30; and the consumption of ultra-high cumulative dose of NSAIDs (ie, a lifetime dose of 5000 pills) was linked to CKD deterioration and mortality.30 However, some long-term studies did not show a direct relationship between NSAID use and the risk of renal function loss.31 ,32 In this study, we found that, after controlling for other confounders, the relationship between NSAID use and the risk of mortality did not reach statistical significance. This could at least be partially explained by the fact that only very few study participants had consumed 5000 or more pills of NSAIDs during the follow-up period.
Limitations
There are several limitations of this study and a causal relationship between the use of non-AA prescribed CHMs and the risk of mortality cannot be inferred based on such an observational study. First, despite the fact that we selected only patients with incident CKD and assessed the effects of non-AA prescribed CHMs use primarily after the diagnosis of CKD to minimise the possibility of selection bias, unforeseen differences in baseline characteristics between patients who received and those who did not receive non-AA prescribed CHMs after the diagnosis of CKD could still exist and account for the study findings.
Second, although we employed Cox proportional hazard regression analysis to control for potential confounders, we did not have data on personal information such as lifestyle, smoking and ethanol drinking, which might confound the relationship between the use of non-AA prescribed CHMs and the risk of mortality.
Third, we did not have access to data on serum creatinine and/or other renal function indices in the NHIRD; therefore, the diagnosis of CKD in this study could only be defined by specific ICD codes. Although we have exercised great caution in defining the diagnosis of CKD, the possibility of coding error in the NHIRD could still be present and might result in an underestimate or overestimate of the relationship between non-AA prescribed CHMs and the risk of mortality. The inclusion of ICD codes not specific to CKD might also lead to a biased estimate. In the sensitivity analysis that limited the study participants to those whose CKD diagnosis was defined by ICD codes 585 and 586; we nevertheless found that the inverse association between non-AA prescribed CHMs and the risk of mortality remained statistically significant.
Fourth, although all CHMs prescribed by certified Chinese medicine practitioners in Taiwan are eligible to be reimbursed by the NHI Bureau, patients with CKD can still obtain non-prescribed CHMs by purchasing the herbs from the herbalists and/or uncertified Chinese medicine practitioners, or gathering CHMs of plant origin by themselves. While we did not have nationwide data on the use of non-prescribed CHMs in Taiwan, which might be hazardous to patients with CKD7 and associated with the use of prescribed CHMs, we did find in a previously published case–control study that only 6.6% of the controls received non-prescribed CHMs.7 Moreover, we did not find any significant relationship between the use of non-prescribed and prescribed CHMs in patients with CKD. Both findings suggested that the use of non-prescribed CHMs was less likely to significantly confound the inverse association between non-AA prescribed CHMs and the risk of mortality observed in this study.
Fifth, adulteration of CHMs by prescription drugs and heavy metals and contamination of CHMs by heavy metals have always been a concern in studying CHMs. In Taiwan, the NHI Bureau mandates all reimbursed CHMs must be manufactured following the ‘Good Manufacturing Practice’. Moreover, the Taiwan Food and Drug Administration (FDA) has conducted annual screening of adulteration of CHMs by prescription drugs and periodically measured the concentrations of various heavy metals in raw materials of CHMs. According to the data reported by the Taiwan FDA, the possibility of adulteration of prescribed CHMs by prescription drugs and/or contamination of prescribed CHMs by heavy metals is negligible in Taiwan.33
Sixth, coding error of prescribed CHMs is possible in a large database like the NHIRD. Although we were unable to check the accuracy of the use of non-AA prescribed CHMs, the information was likely to be accurate and complete. In Taiwan, every CHM prescription is strictly regulated and only recorded prescriptions are eligible to receive reimbursement from the Taiwan NHI Bureau.
Seventh, because there were numerous types of analgesics and NSAIDs and we did not have information on over-the-counter analgesics/NSAIDs, the total number of analgesic/NSAID pills received by patients with CKD in this study might not fully capture the exact dose–response relationship between analgesics/NSAIDs and the risk of mortality in patients with CKD. Still, we did find a positive association between the use of analgesics and the risk of mortality in the multivariate analysis.
Finally, because the types of non-AA prescribed CHMs are extremely diverse, we did not examine the specific ingredients of various compound CHM formulas. Thus, we were unable to identify the specific non-AA prescribed CHM (eg, Angelica sinensis and Astragalus membranaceus) that was associated with reduced risk of mortality in patients with CKD. Moreover, the lack of information on the cumulative dose of each non-AA prescribed CHM prohibited us from conducting further evaluation of any dose–response relationship between specific prescribed CHMs and the risk of mortality.
In conclusion, we found that the use of non-AA prescribed CHMs after the diagnosis of CKD yet before the start of erythropoietin therapy was related to a lower risk of mortality. Further prospective studies are warranted to see whether such an association is truly causal and to identify the specific CHMs that may be beneficial to patients with CKD. Laboratory analysis of the prescribed CHMs to identify exactly which CHMs are in each compound formulation may also be needed. Moreover, it is of great interest to evaluate whether there will be any herb–drug interactions between the use of non-AA prescribed CHMs and western medicine among patients with CKD.
References
Footnotes
-
Contributors CFH, SLH, CCC and CCY conceived of the study. CFH and CCY conducted the analyses. CCY led the writing of the manuscript. All authors commented on the analytic plan and interpretation and contributed to the editing and final approval of the manuscript.
-
Funding This work was supported by a grant (LS-2012-09) from Taiwan Landseed Hospital.
-
Competing interests None.
-
Ethics approval The study protocol was approved by the Landseed hospital institutional review board (IRB-12-23).
-
Provenance and peer review Not commissioned; externally peer reviewed.
-
Data sharing statement The dataset of this study was obtained from Taiwan National Health Insurance Research Database.