Article Text
Abstract
Background Pharmacists are vital to the multidisciplinary care for transplant patients, but the workforce of their work in China remains unclear.
Objective To assess the current status of transplant pharmacists' work in China through a national workforce survey to evaluate service provision across transplantation stages and pharmacist capacity.
Design, setting and participants Nationwide cross-sectional questionnaire-based study (25 March–1 April 2024) involving 91 transplant centres performing>90% of China’s organ transplants.
Main outcomes and measures Pharmacy items and duration provided to patients by pharmacists at each transplant centre and used to calculate full-time equivalents (FTEs).
Results Service provision varied substantially across centres, with only 0.8 pharmacists per 100 transplant patients nationally. Current workforce require 2.6 FTEs/100 transplants for comprehensive services, reducible to 1.2 FTEs if non-clinical duties are excluded. Key challenges included service inconsistency, limited patient coverage and inadequate standardisation/digitalisation. Primary barriers were excessive non-service workloads and insufficient performance incentives. Addressing these issues is an effective way to change the current status of pharmacy services for transplant recipients in China.
Conclusions A national workforce survey revealed significant gaps in transplant pharmacy services in China, particularly in standardisation and digitalisation. To address these issues, key steps include reducing non-essential workloads for transplant clinical pharmacists, enhancing performance incentives, expanding the number of transplant pharmacists and developing standardised service protocols.
- Health Surveys
- Pharmacists
- Health Services
Data availability statement
Data are available upon reasonable request. The data sets generated and/or analysed during the current study are not publicly available considering the privacy but are available from the corresponding author on a reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Nationally representative data from 91 hospitals covering >90% of China’s transplant volume.
Combined structured quantitative metrics (eg, full-time equivalent calculations) with qualitative open-ended questions.
Diversity and complexity of clinical practices may extend beyond the scope captured.
Introduction
Solid organ transplantation is a well-established treatment option for patients with end-organ dysfunction.1 A 10-year update report provides robust evidence highlighting the substantial survival benefits of solid organ transplantation, while also demonstrating the measurable progress in clinical outcomes of organ transplantation since 2012.2 This progress owes much to the implementation of a multidisciplinary approach to post-transplant care, wherein transplant pharmacists play a pivotal role.3–6
In 2020, the American Society of Health-System Pharmacists (ASHP) issued comprehensive guidelines regarding pharmacy services for solid organ transplants.7 These guidelines underscored the indispensable role of transplant pharmacists or pharmacologists within transplant centres, emphasising their necessity for meeting accreditation standards. Moreover, the guidelines elucidated the operational model for transplant pharmacists, providing clarity on their functions and responsibilities within the transplantation process.
Despite the progress seen in many countries, transplant pharmacy services in China are still in their infancy, characterised by ongoing exploration and development.8 As the demand for transplant pharmacists to actively participate in transplant care grows,4 there has been a corresponding increase in the number of professionals in this field, leading to a diversification of pharmaceutical services provided.
Nevertheless, there remains a crucial gap in understanding the current landscape of transplant pharmacy services in China.8 To date, there has not been a nationwide survey conducted to comprehensively assess the number of transplant pharmacists and the scope of pharmaceutical services being offered. Furthermore, a unified set of practice standards outlining the roles and responsibilities of transplant pharmacists throughout the continuum of care is lacking. However, due to differing national contexts, guidelines from other countries only serve as a point of reference and may not be directly applicable.
Therefore, it is imperative to gain a thorough understanding of the current status of transplant pharmacists and their working patterns in China. Therefore, the primary objective of this survey was to conduct a comprehensive assessment of the current situation of transplant pharmacists in China. This research aims to provide a reference and foundation for the development of standardised practices in the field. Additionally, it seeks to assist various programmes in evaluating their transplant pharmacy services and staffing.
Methods
Survey design
The questionnaire (online supplemental material 1) was developed by all executive committee members of the National Alliance of Transplant Pharmacists through multiple online meetings. The alliance, established on 18 December 2022, with the mission of enhancing pharmacists' capabilities in medication therapy management for solid organ transplant recipients, ensuring medication safety and improving patients’ quality of life. Initially comprising 69 members, the alliance had grown steadily by addressing the clinical demands of China’s transplant medicine, aligning with the patient-centred development trend of pharmaceutical care and leveraging policy support. Through academic collaboration, standardisation initiatives and educational outreach, it expanded to 88 members by October 2024. These members are distributed across 64 transplant centres in 25 provincial-level administrative divisions (73.5%, 25/34) in China.
Supplemental material
All opinions were collected and collated by the working group, which consisted of one executive committee member and her students. The objectives of the survey are set out below, with a focus on the first four:
Describe the demographic information of transplant pharmacists currently practising in China.
Obtain detailed information about the daily practice activities of transplant pharmacists in most hospitals in China during all phases of transplantation (ie, evaluation phase, transplant phase and the post-transplantation phase (ambulatory)), as well as activities outside of transplant patient care (eg, pharmacy management, teaching and scientific research).
Obtain the opinions of transplant pharmacists on the current daily practice of transplant pharmacy services.
Calculate the current full-time equivalents (FTEs) of transplant pharmacists in China using the survey data.
The questionnaire was structured into four sections: basic information, including age, education background, job title and name of organisational unit; daily practice activities of transplant pharmacists, referring to the ASHP Guidelines on Pharmacy Services in Solid Organ Transplantation7 (there is no relevant guideline in China), and we have divided the pharmacy services into three phases, including evaluation phase, transplant phase and post-transplant phase (ambulatory), covering tasks such as assessment of medication adherence, reviewing and adjusting prescriptions, conducting pharmacy visits, providing patient education and managing transplant pharmacy clinics; pharmacy management, teaching and research work—these activities were defined as non-transplant specific duties, reflecting the institutional role of the pharmacist rather than the direct provision of transplantation services. This distinction is consistent with the current scope of pharmacy practice in most Chinese hospitals. In addition, transplant pharmacists’ opinions on current pharmacy services. The questionnaire incorporated both qualitative and quantitative questions, including single-choice and multiple-choice questions, as well as open-ended questions. Following several rounds of revisions, a small number of questionnaires were distributed to members within the National Transplant Alliance for a pilot test. After reviewing the data and feedback from respondents, further revisions were made, resulting in the completion of a final draft. The ultimate survey obtained executive approval from the National Transplant Pharmacists Alliance.
Survey participants and dissemination
To ensure data representativeness and maximise participation, the survey targeted transplant pharmacists from all hospitals within the National Transplant Alliance, as well as the top 50 hospitals in China based on transplant volume, totalling 91 hospitals. Data indicate that transplants conducted in these top 50 hospitals encompass 90% of all transplant recipients in China.9 Therefore, the number of organ transplants performed in the hospitals surveyed exceeds 90% of the total transplant volume in China. One transplant centre discussed among themselves to submit only one questionnaire that was reflective of their hospital’s pharmacy services. Employing the online questionnaire method (https://www.wenjuan.com/s/UZBZJvxSu9B/), the survey questionnaires were disseminated via the WeChat groups of National Transplant Alliance, with respondents independently completing them. For transplant pharmacists who were not part of the alliance, we contacted either the director of the pharmacy department or the transplant pharmacist at that transplant centre directly via WeChat, seeking their help in completing the questionnaire and adding the transplant pharmacist’s WeChat for follow-up reminders. The questionnaire was launched on 25 March 2024, and the working group closely monitored participation. After a week-long period (25 March 2024–1 April 2024), non-responsive instances prompted the creation of a follow-up initiative, wherein a member of the working group directly solicited participation from pharmacists in identified hospitals. After an additional 3 days, the survey concluded.
Patient and Public Involvement
It is important to state that patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Data analysis
Data from all respondents were saved using Microsoft Excel, excluding transplant pharmacists who did not provide direct pharmacy services. Descriptive statistics were used to summarise demographic and practice activities. Based on the number of kidney and/or pancreas transplants in the hospitals in 2023 (if kidney and/or pancreas transplantation is not performed, sort by liver transplantation, heart transplantation and small bowel transplantation), grouping hospitals by capacity volume and comparative analyses of quantifiable indicators were performed using SPSS V.22. Continuous data were expressed as means (SD) or medians (IQR), and group comparisons were executed using the t-test. Categorical variables were described as frequencies and percentages, with between-group comparisons performed using χ2 tests. A p value of <0.05 was considered statistically significant. In addition, the formulas for FTEs are as follows:
Actual Transplant Pharmacist Ratio = (Total Number of Transplant Pharmacists/Annual Total Number of Transplants) × 100
FTEs = Actual Working Hours/Standard Working Hours = (Daily Transplant Pharmacy Service Duration+Pharmacy Management Duration+Teaching Activities Duration+Research Activities Duration)/Standard Working Hours (8 hours).
Daily Patient Pharmacy Service Duration = [(Sum of estimated time for all transplant pharmacy service items per patient since admission×Average Hospital Stay Duration of Patients) × Annual Total Number of Transplants] / 1 year (365 days)
Results
A total of 91 hospitals participated in the survey (response rate of 100.0%), of which 13 hospitals (14.3%) indicated that they have not performed organ transplants in recent years. Therefore, our survey was conducted in the remaining 78 hospitals. Additionally, nine hospitals (9/78, 11.5%) did not have a transplant pharmacist involved in the care of transplant patients. It was noteworthy that eight of these nine hospitals were among the top 50 hospitals in China in terms of the number of transplants performed.
Participant demographics
There were a total of 97 transplant pharmacists in these 78 hospitals, with a median age of 36 years (IQR 33–39 years). The majority of pharmacists (74, 76.3%) held postgraduate degrees and intermediate technical titles (59, 60.8%). On average, they had 5 years of clinical employment (IQR 2–7 years), with 32 out of 97 pharmacists (33.0%) having over 5 years of experience. Additionally, 27 pharmacists (27.8%) indicated that they held teaching qualifications in clinical pharmacy and had been qualified for an average of 4 years (IQR 2–6 years). Most transplant pharmacists provided care for the kidneys (87, 89.7%), followed by the liver (68, 70.1%), heart (33, 34.0%), lungs (26, 26.8%) and intestines (3, 3.1%). It was also noted that a majority of respondents reported caring for more than one organ type (65, 67.0%), shown in table 1.
Basic information of transplant pharmacists
Pharmacist activities during the phases of transplantation
Evaluation phase
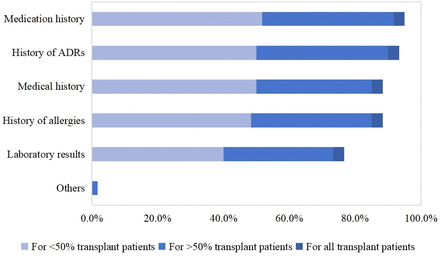
60 out of 78 hospitals (76.9%) perform medication reconciliation on their patients on first encounter. The specific elements involved in medication reconciliation are illustrated in figure 1. Nearly half of the respondents (32/60, 53.3%) indicated that medication reconciliation was conducted at least twice during a patient’s hospital stay. The main focus areas during medication reconciliation include medication history (57/60, 95.0%), history of adverse reactions (56/60, 93.3%), medical history (53/60, 88.3%), allergy history (53/60, 88.3%), laboratory results (46/60, 76.7%) and others (1/60, 1.7%). However, more than half of the hospitals (32/60, 53.3%) stated that they are only able to provide such services to less than half of the patients. Additionally, 26 hospitals (26/60, 43.3%) mentioned that they provide medication reconciliation services to more than half of their transplant patients, while only 2 hospitals (2/60, 3.3%) stated that they provide medication reconciliation services to all patients. The majority of hospitals (37/60, 61.7%) indicated that there is no standard template for recording medication reconciliation, and 78.3% hospitals (47/60) were unable to integrate the appropriate content into the hospital information system.
Percentage of hospitals providing activity during transplant evaluations phase. ADR, adverse drug reaction.
More than half of the transplant centres (42/78, 53.8%) reported assessing patients for medication adherence, with the most frequently used scale being the Morisky Adherence Scale (33/42, 78.6%).10 Additionally, 17 transplant centres (17/42, 40.5%) reported assessing patients only once, mostly during hospitalisation. In total, 7 hospitals (7/42, 16.7%) assess patients’ adherence twice, mainly during the patient’s hospitalisation and at the time of the patient’s discharge; 10 hospitals (10/42, 23.8%) assess patients three times, at the first time of seeing the patient, during the patient’s hospitalisation and at the time of the patient’s discharge; 8 hospitals (8/42, 19.1%) assess patients throughout the whole process, at the first time of seeing the patient (pre-hospitalisation or clinic prior to transplant), during the patient’s hospitalisation, at the time of the patient’s discharge and the later follow-up visit, at a minimum of four times.
Transplant phase
Of all respondents, 60 hospitals (60/78, 76.9%) indicated that they provided pharmacy services during the transplant phase. Figure 2 depicts the pharmacist’s activities during the transplantation phase, focused on individualised pharmacy services (57/60, 95.0%), pharmaceutical ward round (57/60, 95.0%), medical order review (56/60, 93.3%) and medication education (53/60, 88.3%). Among hospitals providing perioperative pharmacy services, only a few respondents (14/60, 23.3%) offer this care to all recipients. The majority of hospitals (23/60, 38.3%) can provide the above pharmaceutical services to less than half of the patients. Additionally, 28 hospitals (28/60, 46.7%) mentioned that they paid extra attention to patients who were retransplanted.
Percentage of hospitals providing activity during transplant phase (individualised pharmacy services: holistic assessment of the patient and customised pharmacy services for the patient).
Of all respondents, 84.6% of the hospitals (66/78) provided therapeutic drug monitoring and 59.0% of the hospitals (46/78) provided pharmacogenetic testing services for the relevant drugs.
Medical order review
Among all the surveyed hospitals, a total of 56 hospitals (56/78, 71.8%) conducted regular medical order reviews. Regarding the frequency of these reviews, most hospitals (33/56, 58.9%) conduct reviews three times per week or more for indications, dosage, repeat medications, drug interactions and contraindications. Additionally, 21.4% (12/56) can conduct reviews at most two times per week, while only 11 hospitals (11/56, 19.6%) indicated daily reviews. In terms of the patient population covered by these services, the majority of hospitals (21/56, 37.5%) could review orders for less than half of their patients, while only 14 hospitals (14/56, 25.0%) mentioned that they review all patients’ orders. Another point worth noting is that among hospitals conducting medical order reviews, only 28.6% (16/56) record the review process and results in the information system.
Pharmaceutical ward round
57 hospitals (57/78, 73.1%) indicated that they conducted regular pharmaceutical ward round for all transplant patients. Regarding the form of these ward rounds, 31 hospitals (31/57, 54.4%) indicated that they use both joint ward rounds with physicians and independent pharmaceutical ward rounds, while 24 hospitals (24/57, 42.1%) conduct the ward rounds in conjunction with physicians. Additionally, only two hospitals (2/57, 3.5%) indicated that the transplant pharmacist conducts solely independent pharmaceutical ward rounds. Regarding the frequency of these ward rounds, 31 hospitals (31/57, 54.4%) mentioned that the frequency for each transplant patient is three or more times per week, 21 hospitals (21/57, 36.8%) indicated a frequency of two or fewer times per week and only 5 hospitals (5/57, 8.8%) conduct daily pharmaceutical ward rounds. Moreover, only 17 hospitals (17/57, 29.8%) mentioned the existence of a standardised template to help record the form and content of the ward rounds, although this template was not uniformly applied across transplant centres. Additionally, 15 hospitals (15/57, 26.3%) indicated that they would record pharmaceutical ward round-related content in the system.
Medication education
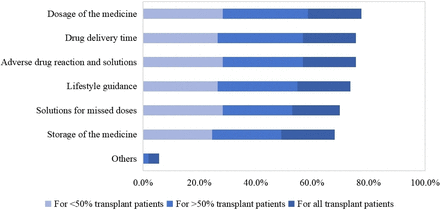
As shown in figure 3, among 53 surveyed hospitals (53/78, 68.0%), respondents indicated that they would provide medication education to their patients, focusing primarily on medication dosage (41/53, 77.4%), drug delivery time (40/53, 75.5%), adverse drug reaction and solutions (40/53, 75.5%), lifestyle guidance (39/53, 73.6%), solutions for missed doses (37/53, 69.8%), storage of medications (36/53, 67.9%) and others (3/53, 5.7%). Regarding the coverage of patient groups by medication education, a large majority of the hospitals (22/53, 41.5%) would provide medication education to more than half of the patients, while 18 hospitals (18/53, 34.0%) would provide medication education to less than half, and 13 hospitals (13/53, 24.5%) would educate all patients. Various educational methods were used, with verbal combined with written or video education being the most commonly used (34/53, 64.2%), followed by solely verbal education (17/53, 32.1%) and and solely written education (2/53, 3.8%). Additionally, 30 hospitals (30/53, 56.6%) indicated that there was a standardised template for documenting medication education, and 14 hospitals (14/53, 26.4%) mentioned that it would be documented in the hospital’s system.
Percentage of hospitals providing concrete activity about medication education during the transplant phase.
Post-transplant phase (ambulatory)
Long-term pharmaceutical management after transplantation was also crucial. However, only 14 hospitals (14/78, 18.0%) indicated that they have developed pharmacist-managed clinics for transplant patients. The average time taken to establish these clinics was 27.4 months (IQR 6.8–34.5 months). Among these clinics, 9 hospitals (9/14, 64.3%) had a standardised template for documenting outpatient pharmacy services and 12 hospitals (12/14, 85.7%) documented the outpatient process in the information system.
Other pharmacist activities
Most hospitals (64/78, 82.1%) indicated they were involved in hospital-wide pharmaceutical management tasks, such as various pharmaceutical data reporting and hospital-wide medical order reviews. The average transplant pharmacist daily time spent on pharmaceutical management tasks was 3.0±1.6 hours. Only one transplant centre expressed having a dedicated pharmacy management team responsible for such matters. Regarding teaching responsibilities, 62 respondents (62/78, 79.5%) participated in teaching jobs, including trainee pharmacist (3/62, 4.8%), pharmacy student (15/62, 24.2%) and those who are both (44/62, 71.0%). The time spent on teaching jobs was 1.5±0.7 hours. Additionally, nearly all respondents (64/78, 82.1%) were involved in scientific research as part of their daily routine, spending an average of 2.0±1.3 hours on research activities.
Pharmacist opinions regarding practice
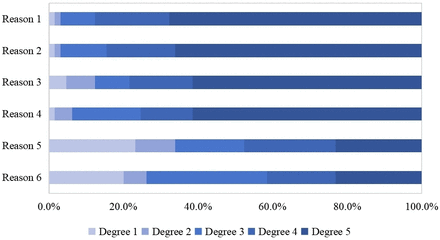
Almost all respondents (71/78, 91.0%) perceived a moderate shortage in the number of transplant pharmacists, with only one respondent perceiving a mild shortage. Six respondents (6/78, 7.7%) denied the lack of transplant pharmacists. Regarding the current state of pharmacy services in China, 65 respondents (65/78, 83.3%) perceived a lack of transplant pharmacy services. The main reasons for the lack of pharmacy services were identified as heavy workload in other daily tasks, lack of performance incentives, the shortage of transplant pharmacists and lack of legal and regulatory support, with degree 5 accounting for more than 60% of the total, especially concerning the high daily workload (degree 5 at 67.7%) (see figure 4).
Participants’ perceptions of specific reasons for the lack of pharmacy services. Note: the higher the degree, the higher the level of consent, for example, ‘degree 1’ means strongly agree and ‘degree 5’ means strongly disagree. Reason 1: heavy workload in other daily tasks; Reason 2: lack of performance incentives; Reason 3: the shortage of transplant pharmacists; Reason 4: lack of legal and regulatory support; Reason 5: insufficient demand from clinicians for pharmacy services; Reason 6: insufficient demand from patient for pharmacy services.
Impact of transplant centre volume on pharmacy services
There were no differences in the average number of transplant pharmacists and the proportion of pharmacy services for patients between transplant centres (table 2). However, in the evaluation phase, as the number of transplants increases, the probability of pharmacists performing medication reconciliation decreases, and the difference was statistically significant (p<0.05).
Impact of transplant centre volume on pharmacy services
Transplant pharmacist FTEs
Based on the existing number of transplants and pharmacists, we had calculated an average of 0.8 pharmacists per 100 transplant patients. To propose the FTE calculation framework based on current practice patterns of Chinese transplant pharmacists, pharmacists need to juggle hospital-wide pharmaceutical management tasks, teaching jobs and scientific research; there was a median of 2.6 transplant pharmacist FTE positions per 100 transplants. It is important to note that the average patient length of stay was 17.2±8.4 days. In contrast, assuming an idealised workflow of transplant pharmacists providing direct pharmacy services and education only, the FTEs would become 1.2 per 100 transplants.
Discussion
This is the first survey of transplant pharmacists in China, which comprehensively described and assessed the daily activities of transplant pharmacists in China, while also calculating the current demand for transplant pharmacists. The data suggested that transplant pharmacy services in China remain underdeveloped.
The importance of transplant pharmacists has been confirmed by many studies3–6; however, there are currently few surveys on the transplant pharmacist workforce. In 2015, a national survey of transplant pharmacists in the USA described the main service activities performed by pharmacists during the different stages of transplantation, highlighting the typical responsibilities of pharmacists practising within the field of transplantation and illustrating that the level of pharmacist involvement varies significantly across transplant centres and stages of transplantation. The survey also calculated that there were currently 1.4 FTE positions for transplant pharmacists per 100 transplants.11 In 2021, a study from Brazil on thoracic transplant12 illustrated that in their transplant centres with pharmacists (n=12), there is not a full-time professional dedicated to their transplant programme, and the activity of transplant pharmacists is significantly lower than that of transplant centres in the USA. It is clear that the activities of transplant pharmacists in the patient care team reflect known benefits. Sam’s systematic review included 12 studies involving 1837 patients, demonstrating that in the solid organ transplant setting, pharmacists can improve medication adherence, reduce medication errors and thereby enhance patient outcomes.4 With the development of transplantation technology, the need for transplant pharmacists will further increase.13
The results of the survey show that there are still many deficiencies in transplant pharmacy services in China, which are mainly reflected in the following areas. First, our research found that there is a significant shortage of transplant pharmacists in China. Our study results indicate that there is currently an average of 0.8 pharmacists per 100 transplant patients, whereas in comparison, the USA has 1.4 FTE positions for transplant pharmacists per 100 transplants, showing a noticeable decrease in numbers.11
Second, we found that even with the presence of transplant pharmacists there are deviations in pharmacy services, with important pharmacy services missing, consistent with foreign studies.11 12 For example, only 53.8% of transplant centres assess patients for adherence, and 48.0% only perform once. Transplant patients require lifelong immunosuppressive therapy, and patient compliance with immunosuppression is closely related to the development of rejection, particularly antibody-mediated rejection and resulting premature graft failure.14 15 This is significantly inadequate compared with pharmacy services in other countries.16–18
At the same time, the number of patients covered by transplant pharmacy services in China is low, with most transplant centres only able to provide pharmacy services to less than 50% of transplant patients.
Lack of standardisation and information technology is also a current problem in China. Standardisation and informatisation are important components of pharmacy services, which are significant for the uniform calculation of what pharmacists do as well as for measuring the value of their work. The clinical pharmacist training programme implemented in China follows a ‘residency pharmacist training+specialised clinical pharmacy training’ model. In most regions of China, pharmacists are required to complete a 3-year residency in pharmacist training. If they wish to pursue a career as clinical pharmacists, they must undergo 1 year of specialised clinical pharmacy training, with transplant pharmacy (or immunopharmacology) being a separate specialty. Hospitals that meet specific requirements can be approved as clinical pharmacy training bases, recruiting clinical pharmacists nationwide according to the approved training direction. In principle, only pharmacists who have obtained clinical pharmacy certification are eligible to practice as clinical pharmacists. However, in the area of transplant pharmacy training, clinical pharmacists in transplant centres are currently trained independently by the transplant centres themselves, with no unified training model. Systematic training in this field is still in its early stages. In the USA, the relevant regulatory agencies (United Network for Organ sharing, Sharing, UNOS and Centers for Medicare & Medicaid Services, CMS)7 have made having transplant pharmacists a mandatory requirement of the transplant patient care team, with clear training modalities,11 and have issued guidelines to guide their daily work. While in China, there is still a lack of corresponding standard documents. Only a small number of hospitals in China had templates for pharmacy services and were able to link to the hospital system, but these templates were developed by individual hospitals and were not standardised, and that in the future standard operating procedures regarding transplant pharmacists should be developed, which can guide and standardise the daily work of the transplant pharmacists.19 At the same time, linking to the hospital system means that the pharmacist’s entire care process can be recorded by the electronic system, making the care process more systematic20; therefore, efforts should be made to form an electronic working system about transplant pharmacists.
Regarding the reasons for the low availability of transplant pharmacist services, a study12 suggested that the main reasons were the low frequency of pharmacist involvement in the transplant team and the low level of training and experience in the field of transplantation. Our study showed that it is mainly the heavy workload in other daily tasks in China, with 67.7% ofrespondents having the highest level of perception for this reason. We revealed that almost all transplant pharmacists in China are currently required to take on work other than patient care, including pharmaceutical routine work, teaching jobs and scientific research, which takes up the vast majority of their working time. In this case, we calculated the FTEs as 2.6; if only pharmacy service-related work is performed, FTEs of transplant pharmacists would be reduced to 1.2 per 100 transplants, close to the findings in the USA. This also confirms the heavy workload of transplant pharmacists in China, especially with other daily work tasks. Therefore, reducing the time spent on other activities and developing a service model of a resident pharmacist would help alleviate the current dilemma.
The next reason was lack of performance incentives, with 66.2% of respondents having the highest rating for this reason. In our survey, we found that pharmacy services for transplant patients are not currently valued by hospitals and the nation, with many transplant centres reporting a lack of performance incentives. However, key performance indicators are essential to enhance the value of pharmacy services, and the implementation of performance appraisal is an inevitable choice for high-quality and sustainable development of hospitals.21 22 Therefore, for pharmacy services, hospitals should develop refined performance appraisal indicators to motivate transplant pharmacists and improve efficiency.
Inadequate pharmacy services are due to lack of pharmacists, as perceived by some transplant centres, with 61.5% of respondents having the highest rating for this reason. In current transplant hospitals in China, based on the existing number of transplants and pharmacists, we have calculated an average of 0.8 pharmacists per 100 transplant patients, while in reality we need 2.6 full-time pharmacists for every 100 transplant patients. Therefore, increasing the number of transplant pharmacists is also a crucial step in improving pharmacy services.
Interestingly, our study found that almost all pharmacists believe that ‘insufficient demand from clinicians or patients for pharmacy services’ is not a factor hindering the provision of pharmacy services. This indicates that both physicians and patients have high expectations of clinical pharmacists as knowledgeable drug therapy experts.
Although this is the largest survey of transplant pharmacists conducted in China, it still has some limitations. First, the participating hospitals may not fully represent all transplant centres in China, potentially limiting the generalisability of our findings. Second, the data collected from pharmacists relied on self-reported measures, including estimated time spent on specific activities, which could introduce recall or social desirability bias. Third, while we investigated common and important aspects of pharmacy services, the diversity and complexity of clinical practices may extend beyond the scope captured in this study. Finally, our analysis did not stratify pharmacy services by specific organ transplant types, despite their potential impact on service demands. Future studies should incorporate organ-specific categorisations and employ objective metrics to validate self-reported data.
Conclusions
Our national workforce survey demonstrates the activities of transplant pharmacists in providing pharmacy services to patients at different stages of transplantation and highlights a significant lack of transplant pharmacy services in China. At the same time, current pharmacy services lack standardisation and digitalisation. To address the current status of transplant recipient pharmacy services in China, reducing other workloads of transplant clinical pharmacist, increasing performance incentives, expanding the number of transplant pharmacists and developing standardised service protocols are crucial steps.
Data availability statement
Data are available upon reasonable request. The data sets generated and/or analysed during the current study are not publicly available considering the privacy but are available from the corresponding author on a reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants. This study was reviewed and formally approved by the Medical Ethics Committee of Beijing Chao-Yang Hospital (no. 2024-科-951). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We thank all pharmacists for their voluntary participation in this study.
Footnotes
HY and XY contributed equally.
Contributors HY was responsible for study conceptualisation and design, data analysis and interpretation, manuscript preparation and manuscript editing. XY was responsible for data acquisition, data analysis, manuscript preparation and manuscript editing. XH was responsible for data acquisition. KM, PC, XL and QQ were responsible for data acquisition, data analysis and manuscript editing. WH and FZ were responsible for data acquisition. GW and RW were responsible for data acquisition and manuscript preparation. ZA was responsible for study design, study conceptualisation, design and interpretation, and is responsible for the overall content as a guarantor.
Funding This work is supported by Beijing Municipal Administration of Hospitals Incubating Program (PG2025003) and Beijing Pharmaceutical Association Clinical Pharmacy Research Program (LCYX-2024-25).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.