Article Text
Abstract
Objectives To assess whether treatment with drugs that activate the Wnt pathway leads to an increased risk of cancer.
Design Systematic review reported using Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) reporting guidelines.
Data sources PubMed, Embase and the Cochrane Library were searched through 1 November 2024.
Eligibility criteria All primary research articles reporting clinical studies, including observational and experimental studies, were included in this review. All studies were eligible for inclusion if they included the exposure of interest, that is, compounds which have been described to activate the Wnt pathway, and the outcome of interest, that is, cancer prevalence. No language restrictions were performed.
Data extraction and synthesis This study was reported according to the PRISMA reporting guidelines. The search string, objectives, and study protocol methods were defined before the study was initiated.
Results A total of 48 studies investigating drugs that activate the Wnt pathway (valproic acid, lithium, cimetidine, olanzapine, clozapine, haloperidol) were included in this systematic review. The results from this systematic review show that, at least for the included compounds in the currently used systemic dosage, cancer prevalence does not significantly increase.
Conclusions The current study found that the use of drugs that activate the Wnt pathway was not associated with an increased risk of cancer. As a promising agent in the regenerative therapy field, further research into Wnt activation as a treatment option should be explored.
PROSPERO registration number CRD42021286193.
- Prevalence
- Epidemiology
- ONCOLOGY
Data availability statement
No data are available.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Inclusion of all study designs, providing a broad overview of studies covering the topic.
Substantial heterogeneity in study designs, inclusion of types of patients and conditions.
We cannot generalise the outcomes based on the broad mechanism of action of the compounds included.
Introduction
The Wnt/β-catenin pathway is a signalling cascade that controls cell proliferation, cell polarity and cell fate determination during embryonic development and tissue homeostasis.1 Wnt/β-catenin signalling is known to be involved in the development of multiple tissues, including brain, eye, ear, spinal cord, bone and cartilage among many others.2 In adulthood, crucial roles in the function of intestine, bone and skin have been described for Wnt/β-catenin signalling.2 Wnts (the ligands that activate the Wnt/β-catenin signalling pathway) are growth stimulatory factors that ultimately lead to cell proliferation. Importantly, dysregulated Wnt signalling has been associated with several diseases such as degenerative diseases,1 neurodegenerative disorders,3–5 schizophrenia,5 ageing-related tissue fibrosis,6 autoimmune diseases7 and many types of cancer.8–12
Currently, targeting the Wnt/β-catenin signalling pathway, either by activating or inhibiting it, is being researched as therapy for some types of cancer,13 14 neurodegenerative diseases15–18 and hair loss.19 20 When therapeutic agents target crucial developmental signalling pathways (such as Wnt, Notch, Hedgehog and bone morphogenic protein pathways) serious and devastating effects on embryogenesis and carcinogenesis might arise due to increased cell proliferation. In line with this, continued activation of the Wnt pathway has been associated with therapy resistance in patients with cancer and has been shown to promote self-renewal of cancer cells.21 Unfortunately, the effect of Wnt activation on cancer prevalence has not been consistently studied. In the last 15 years, common drugs used in the clinic have been described to activate the Wnt pathway.22 23 The most common Wnt activators used in the clinic are lithium and valproic acid (VPA), which have been used as treatment for psychiatric disorders since the 1960s.24–26 Besides, many novel therapeutic drugs have been synthesised specifically to activate Wnt in the last 10 years and are used in the clinic.27 Many of these drugs activate the Wnt signalling pathway through the inhibition of glycogen synthase kinase 3 (GSK3).28 This is one of the most well-studied mechanisms for activating the Wnt signalling pathway.28
There are many novel therapeutic drugs in development for clinical usage that activate the Wnt pathway. However, safety concerns regarding its activation remain.29 Therefore, we conducted a systematic review to address the association between the use of drugs that activate the Wnt pathway and the prevalence of any type of malignancy in the clinic. Our aim was to assess whether treatment with drugs that activate Wnt leads to an increased risk of cancer.
Methods
We evaluated all data available on clinical use of Wnt activators following the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) 2020 writing guideline for systematic reviews.30 Patient, Intervention, Compariron, and Outcome (PICO) framework was used to improve the search strategy.31 The outcome of interest was the prevalence of any cancer, malignancy or neoplasm, regardless of age, sex and geographical location. The exposure of interest was any compound activating the Wnt pathway, regardless of indication, dosage and duration. An overview of the included compounds and their mechanism of action is available in table 1.
Mechanisms of action of all drugs included
Search strategy
The final search was done on 1 November 2024. PubMed, Embase and Cochrane databases were searched. All articles until 1 November 2024 were included in the search. On Embase, conference abstracts and reviews were removed. No further search filters were used. No language restrictions were applied. The search syntax consisted of names of medication with known Wnt activating properties used in the clinic combined with synonyms for ‘cancer’. The full search strategy can be found in online supplemental table S1.
Supplemental material
Article selection
All primary research articles reporting clinical studies, including observational and experimental studies, were included in this review. Studies were eligible for inclusion if they included the exposure of interest, that is, compounds which have been described to activate the Wnt pathway, and the outcome of interest, that is, cancer prevalence. Patients of all ages were eligible for this study. No control group was required. Articles assessing compounds with no clear Wnt activating properties were excluded. Animal studies, in vitro studies and non-primary research articles like review articles and letters were excluded. Two independent reviewers (AA, GF, NS-C, STB) screened titles and abstracts of collected studies after duplicate removal for eligibility criteria. Discrepancies were resolved by discussion between the two reviewers until a consensus was reached. Full-text screening was performed by two independent reviewers, and disagreements were solved as above. Rayyan systematic review tool32 was used to semi-automate the primary screening.
Data extraction
A data extraction table was used to extract study characteristics and findings by two reviewers (AA and NS-C) with the software Microsoft Excel. Data extraction was performed by one reviewer and checked by another reviewer. Discrepancies were solved by discussion between the two reviewers until a consensus was reached. The data extraction table included the following information: Study, indication for intervention, population, age, geographical location, used Wnt activator, used control group, cancer prevalence and cancer type (online supplemental tables S2–S10). No authors were contacted due to data unavailability after inclusion.
Supplemental material
Supplemental material
Critical appraisal
The methodological quality of included articles was assessed by two reviewers (AA and NS-C) using the Newcastle-Ottawa Scale (NOS) for non-randomised studies as a reference guide.33 Risk of bias assessment was performed by one reviewer and checked by another reviewer. Risk of bias in cohort studies was assessed for the following domains: selection bias, comparability of cohorts and outcome (online supplemental tables S11–S18).
Effect measures
Results were expressed according to the reported ratios from the published studies. This includes percentages, ORs, risk ratios (RR) and HRs, in accordance with study design and available data. When unavailable, RRs and ORs were calculated. All ratios were used to answer the main questions qualitatively. No quantitative analyses were conducted for this systematic review.
Study registration
PROSPERO, CRD42021286193.
Patient and public involvement
None.
Results
Article selection
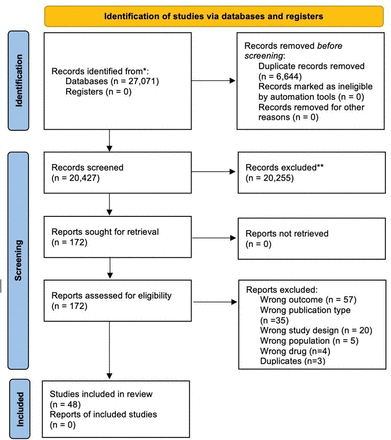
Our PubMed database search until November 2023 yielded a total of 25 969 articles. After duplicate removal, 20 427 articles remained, which were screened for title and abstract. Following title and abstract screening, 172 articles were eligible for full-text screening. All 172 articles could be retrieved. After full-text screening, 48 studies were included for this review. Main reasons for exclusion were outcome that was not in our inclusion criteria, publication type, study design, population and different drug. Article screening is summarised in the flowchart in figure 1.
Article selection flow diagram. The identification of studies via databases and registers is presented above. The selection was divided into three stages. Identification in databases and registers. Then screening and lastly inclusion. The protocol was performed based on the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) 2020 flow diagram for new systematic reviews which included searches of databases and registers only.
Study characteristics
Included studies, which are summarised in online supplemental tables S2–S10, consisted of 21 cohort, 19 case–control and 8 pharmacovigilance studies. Drugs with reported Wnt activating properties included were VPA (13 studies), lithium (15 studies), haloperidol (7 studies), cimetidine (10 studies), clozapine (9 studies) and olanzapine (7 studies). Some studies assessed multiple drugs of interest.
Studies were performed in multiple countries, including multiple European and Asian countries in addition to the USA. Additionally, a WHO pharmacovigilance database consisting of 160 countries was included.34 Most common indications were psychotropic, gastrointestinal and neurological use. All compounds were administered systemically in clinical dosing. Most studies assessed any type of cancer prevalence. All studies assessed cancer risk by analysing clinical data or performing questionnaires. In addition, a few studies included histological verification for cancer diagnosis in addition to clinical data.35–38 All Wnt activating compounds were used in their clinical dose respective to their indication.
Risk of bias
Based on the NOS, all but one included study concerning VPA were determined to have a low risk of bias (online supplemental tables S11 and–S12). One study by Stritzelberger et al (online supplemental table S12) did not show all data concerning VPA.39
Concerning lithium, for both cohort and case–control studies, most studies were determined to have low risk of bias (online supplemental tables S13 and S14). One cohort study by Zaidan et al (online supplemental table S13) and three case–control studies by Hallas et al, Kahan et al and Tamim et al (online supplemental table S14) were subject to a high risk of bias.40–43
Most studies reporting cimetidine use had a high risk of bias (online supplemental tables S15 and S16). Main points were missing data, lack of control group or no comparability of groups. The cohort study by Velicer et al (online supplemental table S15) was determined to be of fair risk of bias.44 Only the study by Rossing et al (online supplemental table S15) was determined to be of low risk of bias.45
For haloperidol, both the cohort study by Wang et al (online supplemental table S17) and the case–control study by Friedman et al (online supplemental table S18) were determined to have low risk of bias.46 47 The risk of bias in the case–control study by Hsieh et al (online supplemental table S18) was high because they used non-gastric cancers as a control for gastric cancer instead of healthy individuals with no cancer.48 The case–control study by Pottegård et al (online supplemental table S18) was determined to be of good quality.37
Outcomes
VPA
Seven cohort studies assessed the association between VPA use and cancer prevalence.35 49–54 Six studies showed no statistically significant difference in cancer prevalence between exposed versus controlled subjects, respectively (50, RR=0.877 (0.642–1.032); 51, RR=1.18 (0.96–1.46); 52, RR=0.848 (0.563–1.277); 54, RR=0.848 (0.563–1.277); 35, HR=0.96 (0.84–1.19), 1.0 (0.8–1.3), 1.0 (0.7–1.3); 53 RR=1 (0.7–1.3)). The study by Chavez et al evaluated melanoma prevalence in VPA-exposed individuals. In this study, VPA-exposed individuals had a significantly reduced prevalence of melanoma compared with controls (49, HR=0.64 (0.51–0.79)).
Additionally, six case–control studies assessed the association between VPA use and cancer prevalence.38 39 43 55–57 All studies showed no statistically significant increase in cancer prevalence between exposed vs controlled subjects, respectively (58, OR=0.85, (0.70–1.04); 43, OR=1.21 (0.95–1.56); 39, p=0760; 55, OR=0.62 (0.42–0.92); 38, 0.2% cases and 0.2% control group; 57, OR=0.58 (0.39–0.56)).
Lithium
Nine cohort studies assessed the association between lithium use and cancer prevalence, including melanoma, urinary tract tumours, malignant neoplasms, invasive breast cancer and any type of cancer.36 42 52 53 58–62 Six studies showed no statistically significant difference in cancer prevalence between exposed versus controlled subjects, respectively (36, OR=1.19 (0.71–2.01); 60, RR=1.01 (0.97–1.05); 62, risk difference = −2.8% (−9.7% to 4.1%) for cohort 1 compared with −3.0% (−6.0% to 0.1%) for cohort 2; 61, RR=1.04 (0.89–1.23); 58, RR=0.92 (0.58–1.46); 53, RR=1 (0.6–1.6)). Asgari et al and Huang et al evaluated cancer risk in lithium-exposed individuals compared with controls. In both studies, lithium-exposed individuals had a significantly reduced cancer risk compared with controls (59, unadjusted HR=0.68 (0.51–0.90); 52, RR=0.426 (0.186–0.975)). Zaidan et al found an increased risk of renal tumours in patients exposed to over 20 years of lithium in comparison to both the general population and to kidney function matched controls (based on glomerular filtration rate), p=0.04.42
Additionally, six case–control studies assessed the association between lithium use and cancer prevalence.40 41 43 57 63 64 Five studies showed no statistically significant difference in cancer prevalence between exposed versus controlled subjects, respectively (41, 0.8% vs 0.9% incidence; 64, OR=1.01 (0.86–1.19) for any use, OR=1.06 (0.84–1.34) for>5 years use; 40, standardised incidence ratio=0.93 (0.6–1.38) for male subjects and 1.25 (0.91–1.69) for female subjects; 63, OR=1.3 (0.7–2.1); 57, OR=0.81 (0.58–1.12)). Hallas et al showed a slight increase in cancer prevalence in subjects with long-term exposure to lithium,43 OR=1.19 (1.03–1.39)).
Cimetidine
Three cohort studies assessed the association between cimetidine use and cancer prevalence.44 65 66 The study by Møller et al did not include a control group.65 The remaining two cohort studies investigated gastrointestinal, breast and prostate cancer risk and found no significant increase in cancer risk in the groups exposed to cimetidine in comparison to controls (44, RR=0.97 (0.61–1.53); 66, RR=0.9 (0.8–1.1) for breast cancer risk in women and RR=0.7 (0.6–0.8) for prostate cancer in men)). Rossing et al found a slightly increased risk of prostate cancer in a subgroup of men who had filled>21 prescriptions of cimetidine,66 RR=1.4 (1.0–1.9)).
Five case–control studies assessed the association between cimetidine use and cancer prevalence.67–71 In all studies, cimetidine exposed individuals showed no significant difference in ratio compared with controls (67, OR=0.9 (0.6–1.2); 68, OR=0.39 (0.17–0.89); 71, ductal carcinoma, ever use: OR=1.1 (0.8–1.5); >2 years use, 0.9 (0.5–1.5); 70, no analysis reported; 69, OR=2.1 (0.7–6.3)). Lastly, a cohort study and a surveillance study conducted by Colin-Jones et al showed no increased cancer prevalence after cimetidine exposure.72 73
Haloperidol
A cohort study by Wang et al assessed the association between haloperidol use and breast cancer prevalence, including a total of 46 269 women. A breast cancer prevalence of 0.052% (1228 cases in 237 242 person-years in the control group and 240 cases in 46 269 person years in the haloperidol group) was found in both exposed and unexposed groups, indicating no significant increase in breast cancer prevalence in women exposed to haloperidol compared with unexposed women.46
Additionally, three case–control studies assessed the association between haloperidol use and cancer prevalence. A case–control study by Friedman et al found a potential negative association between haloperidol use and prostate cancer risk, compared with controls depending on duration,47 at>2 years of use, OR=0.54 (0.20–1.44), at>1 year of use, OR=0.32 (0.12–0.84); at <1 year of use, OR=0.69 (0.48–0.99). Another case–control study by Hsieh et al found a reduced risk of gastric cancer associated with haloperidol use,48 OR=0.25 (0.14–0.46). A third, population-based case–control study by Chen et al assessed the risk of endometrial cancer after exposure to haloperidol and other antipsychotics. For haloperidol, an increase in endometrial cancer after exposure to haloperidol was found,74 OR=1.75 (1.31–2.34).
Three database studies assessed the association between haloperidol use and cancer prevalence. The database study by Maeshima et al using the Japanese adverse drug event database showed no increased risk of breast cancer in women exposed to haloperidol,75 reporting OR (ROR)=0.49 (0.07–3.51). However, the study by Lertxundi et al using the European pharmacovigilance database showed a possible increased risk of pituitary tumours of subjects exposed to haloperidol,76 Proportional Reporting Ratio (PRR)=7.0 (4.35–11.3). Finally, a pharmacovigilance study using the adverse event reporting database from the US Food and Drug Administration by Szarfman et al suggested a possible increased risk of pituitary tumours in patients exposed to haloperidol,77 Absolute Risk Reduction (ARR)=5.6 (2.9–13).
Olanzapine
Three case–control studies assessed the association between olanzapine use and cancer prevalence. A nationwide case–control study by Pottegård et al assessed the association between olanzapine use and breast cancer prevalence. Breast cancer cases were verified by histology. This study found a slightly increased risk of oestrogen receptor-positive breast cancer in subjects exposed to olanzapine, attributed to its prolactin-elevating properties when the study was only adjusted for age and gender,37 adjusted OR (aOR)=1.30; 95% CI=1.09 to 1.56); however, in the fully adjusted model, no significant increase was found (aOR=1.15; 95% CI=0.9 to 1.47). Another case–control study by Hsieh et al found a reduced risk of gastric cancer associated with olanzapine use48 (OR=0.13 (0.05–0.35)). Lastly, the case–control study by Chen et al found no increased risk of endometrial cancer after exposure to olanzapine74 (OR=1.14 (0.56–2.30)).
Three database studies assessed the association between olanzapine exposure and cancer prevalence.75–77 The database study by Maeshima et al showed no increased risk of breast cancer in women exposed to olanzapine75 (ROR=0.51 (0.07–3.51)). However, the database studies performed by Lertxundi et al and Szarfman et al suggested an increased risk of pituitary tumours of subjects exposed to olanzapine76 (PRR=2.53, (1.57–4.1))77; ARR=2.3 (1.4–3.7)).
Clozapine
One cohort study by Tiihonen et al assessed the risk of developing haematological malignancies after exposure to clozapine. A significant, dose-dependent, increased risk of haematological malignancies was found78 (aOR=3.35 (2.22–5.05) for>5000 defined daily dose cumulative exposure). Four case–control studies assessed the association between clozapine exposure and cancer prevalence. The case–control study by Hsieh et al assessed the association between clozapine exposure and cancer prevalence and found a reduced risk of gastric cancer associated with clozapine use48 (OR=0.35 (0.13–0.97)). The case–control study by Chen et al found no increase in endometrial cancer risk after exposure to clozapine74 (OR=1.14 (0.56–2.30)). The case–control study by Tiihonen et al found an increased risk of haematological malignancies after exposure to clozapine78 (aOR=2.94 (2.07–4.17)). Interestingly, no significant difference for non-haematological malignancies was found78 for clozapine (aOR=1.47 (1.25–1.47)); as compared with other antipsychotics: (aOR=1.30 (1.15–1.47)). Finally, the case–control study by Brainerd et al also found an increased prevalence of haematological malignancies after clozapine exposure in war veterans79 (OR=1.31 (1.08–1.60)).
Additionally, five database studies assessed the association between clozapine exposure and cancer prevalence. Two database studies by Szarfman et al and Lertxundi et al, assessed the association of clozapine and pituitary tumour prevalence.76 77 For clozapine, both studies showed no significant increase in pituitary tumour prevalence in subjects exposed to clozapine77 (ARR=0.9 (0.4–1.7))76; (PRR=0.98 (0.5–1.8)). Two pharmacovigilance studies by Chrétien et al and Dawson et al assessed the risk of developing haematological malignancies in subjects exposed to clozapine, due to the risk of severe haematological side effects when using clozapine.34 80 In the first study, clozapine-exposed individuals had a significantly increased prevalence of leukaemia aOR=3.54 (2.97–4.22) and malignant lymphoma, aOR=9.13, (7.75–10.77) compared with controls.34 In the second study, an excess of haematological malignancies in subjects exposed to clozapine was reported, indicating a possible increase in cases (no analysis performed).80 Finally, a database study by Uwai and Nabekura assessed the risk of non-haematological malignancies in subjects exposed to clozapine.81 The study showed a possible relationship between clozapine and multiple non-haematological malignancies including lung, gastrointestinal, oesophageal, throat malignancies and metastases to the spine81 (ROR=1.28 (1.22–1.34)).
Discussion
The aim of this review was to assess the risk of cancer development after the use of drugs that activate the Wnt pathway in humans. 48 observational studies (online supplemental tables S2–S10) analysing the risk of cancer of six different drugs that have known Wnt activating properties were included in this systematic review. The drugs assessed in this review were VPA, lithium, cimetidine, haloperidol, olanzapine and clozapine. Most of the included studies showed no increase in cancer prevalence after being exposed to Wnt activating drugs. Most notably, there are the 18 included cohort studies, which were assessed to have low risk of bias. These studies showed no increased cancer prevalence, and in some cases even a decreased cancer prevalence. These results suggest that using medication that activates the Wnt pathway in patients does not elevate cancer prevalence.
A few included studies showed an increase in the prevalence of malignancies after usage of Wnt activating drugs. Interestingly, the included studies that showed an increase in cancer prevalence reported increased cancer prevalence for specific cancer types; there was not a systematic increase in cancer risk. The study by Zaidan et al, showed an increased risk of developing solid renal tumours after a median of 20 years of lithium exposure. However, as lithium is known to be nephrotoxic, and no systemic increase in cancer risk was observed, this increase in cancer prevalence could be attributed to direct toxicity, rather than the activation of the Wnt pathway.42 Chen et al found an increased risk of endometrial cancer after exposure to haloperidol, attributed to antipsychotic-induced hyperprolactinaemia, which is a common side effect of antipsychotics, and not to the Wnt pathway activation. Of note are both olanzapine and clozapine, which also activate the Wnt pathway, but showed no increase in endometrial cancer risk.74
One study (which had many confounders and a high risk of bias) found an increased prevalence of gastric cancer in patients who had used cimetidine for gastric ulcers compared with the general population.73 No other included studies reported an increased cancer risk after cimetidine exposure. Therefore, it is not likely that cimetidine is carcinogenic. In this context, patients with gastric ulcers are already at a higher risk of developing gastric cancer.82 A better control for this study would have been patients with gastric ulcers and no cimetidine use.
Lastly, and most notably, multiple studies found an increased prevalence of haematological malignancies in subjects that were exposed to clozapine.34 78 80 Clozapine is well-known as the first second generation (atypical) antipsychotic and gold standard drug for treatment-refractory schizophrenia, but it has many adverse effects. Agranulocytosis is a relatively common and well-known side effect of clozapine.83 Bone marrow toxicity has been described in in vitro studies.84 The pathogenesis of clozapine-induced agranulocytosis or bone marrow toxicity is still not clear; however, it is unlikely to be Wnt associated. Multiple alternative hypotheses have been described,83 all non-related to the Wnt pathway activation. In the case–control study performed by Tiihonen et al, they reported no differences in non-haematological cancer risk for clozapine in comparison to other antipsychotic drugs.78 Based on available data, we can conclude that subjects exposed to clozapine are at an increased risk of haematological cancers, due to direct bone marrow damage, unrelated to its Wnt pathway activating properties. The fact that the increased cancer risk in patients exposed to clozapine has only been found in haematological malignancies and not in solid tumours supports this hypothesis.
In addition to cohort and case–control studies, multiple pharmacovigilance/surveillance studies were included in this systematic review (online supplemental tables S2–S10). The pharmacovigilance/surveillance studies by Lertxundi et al and Szarfman et al showed an increased risk of developing pituitary tumours after being exposed to the antipsychotics haloperidol and olanzapine.76 77 Nonetheless, this risk was attributed to antipsychotic-induced hyperprolactinaemia, which is a common side effect of antipsychotics, and not to the Wnt pathway activation. None of the included studies showed an increased risk of non-pituitary malignancies. Therefore, we can conclude the increase in cancer risk is not caused by the Wnt activating properties of these drugs.
Strengths and weaknesses of the review
We assessed the cancer risk of multiple drugs with laboratory proven Wnt pathway activation. Most of the included drugs activate the Wnt pathway through GSK3-Beta inhibition (table 1).85 86 Since the activation of Wnt is not their main therapeutic target, the level of Wnt activation may differ between various drugs. However, to assess all data available on the prevalence of cancer after usage of drugs that activate Wnt, we included all available mechanisms to Wnt activation. This study therefore included all papers available.
This systematic review included a complete search of all data available until 1 November 2024. Moreover, bias was minimised by using two independent authors in the screening process.
Strengths and weaknesses of the included studies
In this review, a total of 48 studies were included, summing up extensive data on multiple drugs activating the Wnt pathway. Notably, 21 cohort studies were included, of which 18 were assessed to be subject to a low risk of bias. This leads to an extensive dataset on the cancer risk of these compounds. Opposed to the cohort studies, however, the 19 included case–control studies involved a wide variety in risk of bias and study methods. Notably, the articles regarding cimetidine, which were relatively old overall, showed a high risk of bias.
The wide variety in study designs, types of patients, cancer types and used compounds, resulting in heterogeneity in the data prevented us from conducting a meta-analysis, which results in limitations in drawing an overall conclusion regarding the cancer risk of Wnt pathway activation.
Another limitation is the drugs that were assessed in the included studies of this review. These drugs activate the Wnt pathway, but they are not specifically designed and used for their Wnt activating properties. These drugs have been in use since the 1950s, and their Wnt activating properties have been described only in the last 30 years, mainly in in vitro experiments. Novel Wnt activating drugs, like CHIR99021,87 have been produced in the past few years. However, given that these drugs have not been used clinically, their risk is not clear and has to be assessed in the future. Furthermore, included studies had considerable missing data, including data to assess dose-related cancer risk, such as duration of treatment and used dosages. In most articles, Wnt activating properties were not discussed. Finally, there were no randomised controlled trials included in this review; only observational studies were included, which are by design more at risk of bias due to the lack of randomisation.
Conclusions
Various applications are being researched for both activating and inhibiting the Wnt pathway. Cancer risk, however, remains a big concern.29 The results from this systematic review show that, at least for the compounds included in the currently used systemic dosage, no increase in cancer prevalence was found in any of the studies included, which could be attributed to Wnt pathway activation. These findings suggest that compounds activating the Wnt pathway are, regarding cancer risk, a safe option.
Before taking this conclusion into medical practice, however, further research on higher dosages, local administration and drugs specifically designed to induce Wnt activation should determine whether the activation of the Wnt pathway is indeed a safe treatment option with regards to cancer risk.
In the regenerative therapy field, Wnt activation is a promising agent for future treatment opportunities. Based on the data in this review, we can conclude that Wnt activation by the assessed compounds leads to no increased cancer risk. Therefore, further research into Wnt activation as a treatment option should be explored.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
References
Footnotes
AA and NS-C are joint first authors.
Contributors AA: conceptualisation, data curation, formal analysis, investigation, visualisation, writing—original draft preparation. NS-C: conceptualisation, data curation, formal analysis, investigation, project administration, supervision, visualisation, writing—original draft preparation, writing—review and editing. GF: data curation, formal analysis, investigation. STB: data curation, formal analysis, investigation. RS: supervision, writing—review and editing. IS: conceptualisation, resources, project administration, supervision, writing—review and editing. LVS: conceptualisation, project administration, supervision, writing—review and editing. NS-C is the guarantor.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.