Article Text
Abstract
Introduction Acute pneumonia (AP) remains a leading cause of death in the older population. Excess risk of death after AP is partly due to cardiovascular (CV) events. We aim to evaluate whether aspirin at a preventive dose (100 mg daily) introduced at the acute phase of AP reduces 90-day mortality.
Methods and analysis The ASPirin for Acute Pneumonia in the elderlY study is a phase III multicentre randomised double-blind, placebo-controlled, superiority clinical trial, which will investigate the efficacy and safety of aspirin in older patients with AP hospitalised in a French university and non-university hospitals. Patients will be randomised in a 1:1 ratio between two groups receiving daily either 100 mg of aspirin or a placebo, within 84 hours following radiologically proven AP diagnosis for 90 days. This study aimed at assessing the efficacy of aspirin on all-cause mortality after AP at 90 days (D90) (primary objective), D30 and D120 after randomisation, CV mortality, major adverse CV events (MACE) (ie, myocardial infarction, stroke, heart failure, new atrial fibrillation and pulmonary embolism, CV death and sudden death) incidence, length of intensive care unit and hospital stay, unscheduled rehospitalisation, dependence, overall and MACE-free survival, as well as safety outcomes (bleeding incidence). The sample size, calculated considering a 90-day mortality of 25% and a reduction of 10% in the aspirin group, a two-sided alpha risk at 5% and power of 80%, is 500 patients to prove the superiority of aspirin over placebo. To account for screening failures and consent withdrawals, 600 patients (300 per arm) will be included.
Ethics and dissemination This study has full approval from an independent Ethics Committee. Participants will sign a written informed consent ahead of participation. Findings will be published in peer-reviewed journals and conference presentations.
Trial registration number EU CTIS: 2024-510811-32-00.
- Mortality
- Aged
- Cardiovascular Disease
- Respiratory infections
- Randomized Controlled Trial
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
All-cause mortality as a clinically relevant primary outcome.
Large population from all medical departments of university and non-university hospitals with broad inclusion criteria, allowing wide generalisation of results.
Inclusion of very old multimorbid patients, usually excluded from clinical trials.
Exclusion of patients under anticoagulant or antiplatelet drugs at admission (bleeding risk).
Delay in acute pneumonia diagnosis and inclusion could decrease prevention of early cardiovascular events.
Introduction
Acute pneumonia (AP) is a major medical issue in the older population. It is the second leading cause of hospitalisation behind heart failure1 and one of the leading causes of death. The mortality rate is estimated at up to 30% in the very old.2 Up to 75% of AP in older patients require hospitalisation.3 Nearly 70 years after penicillin was discovered, no major therapeutic progress has been made regarding the medical care of AP, and the mortality rate has not decreased significantly.4 5 In the older population, there is an urgent need to improve the long-term prognosis of this frequent and severe disease. These deaths occur despite the fact that the causative bacteria are largely eradicated from tracheal secretions and the bloodstream in the first 24 hours of antibiotic therapy.6 If the choice of appropriate antibiotic therapy is a key issue in the acute phase of AP, the long-term prognosis in very old patients mainly depends on comorbidities’ decompensation.7 Excess risk of mortality persists for several months after AP,8 mainly due to cardiovascular (CV) events.9 10
The prothrombotic effects of AP pathogens have been demonstrated in several experimental studies.11–13 Recent literature increasingly highlights a significant association between cardioprotective drugs and outcomes after AP.14–17 Among them, statins, β-blockers, ACE inhibitors and angiotensin II receptor blockers have been associated with decreased short-term and long-term mortality.14 15 Antiplatelet therapy is increasingly associated with improved outcomes after AP. In addition to its antithrombotic effects, aspirin has been involved in the adaptive immune response and could play a role in the interactions with microorganisms.18 Falcone et al reported that all-cause mortality was reduced by one-half with a preventive dose of aspirin after propensity score weighting in a large retrospective study of older patients with AP.19 Recently, Rögnvaldsson et al found similar results in a nationwide study of bacteremic pneumococcal pneumonia in Iceland.20 Such findings have not been confirmed in the ANTISEPSIS primary prevention study.21 In this large randomised trial including 16 703 community-dwelling adults without major illness, aged 70 or more, before any infectious event, daily low-dose aspirin treatment failed to reduce deaths associated with sepsis (predominantly linked to AP). However, whether aspirin treatment introduced at the AP diagnosis prevents CV events and mortality remains to date poorly evaluated. Only one randomised open-label trial addresses this question, demonstrating a significant reduction under aspirin in the incidence of CV deaths in patients aged 67 in mean with AP and CV risk factors.22 Older patients are at high risk of AP but also of post-AP CV events.23 However, no studies have evaluated the efficacy of aspirin prevention in a population of older patients hospitalised for AP. This older population, often neglected by medical research, represents a growing portion of the hospitalised population in industrialised countries.
We hypothesise that aspirin reduces all-cause mortality after AP in an old multimorbid population, particularly at risk of CV complications. In this randomised, controlled, multicentre trial, we aim to evaluate the clinical impact of aspirin 100 mg per day during 90 days on all-cause mortality at 90 days, in patients aged ≥75 years hospitalised for AP.
Methods and analysis
Design and setting
The ASPirin for Acute Pneumonia in the elderlY (ASPAPY)study is a phase III multicentre randomised double-blind, placebo-controlled clinical trial, with a superiority design which will investigate the efficacy and safety of aspirin in older patients with AP. Patients will be randomised in a 1:1 ratio between two groups receiving either 100 mg of aspirin or an identical-aspect placebo, within 84 hours following AP diagnosis, for 90 days.
15 university and non-university hospitals in France are scheduled to participate in the ASPAPY study.
Study objectives
The main objective is to assess the efficacy on all-causes mortality after AP at 90 days (D90) after randomisation.
Secondary objectives will assess (a) the efficacy of aspirin on (1) all-cause mortality at D30 and at D120; (2) CV mortality (ie, mortality related to major adverse CV events (MACE)) at D30, D90 and D120; (3) MACE incidence within 30 days, 90 days and 120 days following randomisation; (4) length of intensive care unit (ICU) stay within 90 days following randomisation; (5) unscheduled rehospitalisation within 30 days and 90 days following randomisation (precluding follow-up and rehabilitation care and long-term care); (6) length of firth hospital stay (precluding follow-up and rehabilitation care and long-term care); (7) dependence at D90 and (8) overall survival and MACE-free survival at D120 and (b) the safety of aspirin.
Endpoints
Primary and secondary outcomes are listed in box 1. The primary outcome is all-cause mortality at D90 after randomisation. D90 endpoint was chosen considering the excess risk of CV events persisting until 90 days after AP in the literature.9 Secondary efficacy outcomes will include all-cause and CV mortality, incidence of MACE, that is, myocardial infarction, stroke, heart failure, new atrial fibrillation, pulmonary embolism, CV death and sudden death, number of days in the ICU, occurrence of unscheduled rehospitalisation, length of first hospital stay, proportion of patients with new institutionalisation and dependence, and overall and MACE-free survival. Safety endpoint will include the frequency of severe and non-severe bleeding events.
Outcomes
Primary outcome
All-cause mortality at D90 after randomisation.
Secondary efficacy outcomes
All-cause mortality at D30 and D120.
CV mortality at D30, D90 and D120 (ie, mortality related to MACE).
MACE include myocardial infarction, stroke, heart failure, new atrial fibrillation, pulmonary embolism, CV death and sudden death.
Incidence of MACE (composite endpoint including at least one of the following: myocardial infarction, stroke, heart failure, new atrial fibrillation, pulmonary embolism, CV death, sudden death) within 30 days, 90 days and 120 days following randomisation.
Number of days in the ICU within 30 days and 90 days following randomisation.
Occurrence of unscheduled rehospitalisation within 30 days and 90 days following randomisation.
Length of first hospitalisation in days (precluding follow-up care, readaptation and rehabilitation services, long-term care facilities).
Proportion of patients newly institutionalised (ie, entering a nursing home) at D90 and proportion of patients with a decrease of ≥1 point between the state before randomisation and D90 on the activity of daily living (ADL) scale.24
Time (in days) to death from any cause, date of point (D120) or date of last news, whichever occurred first.
Time (in days) to MACE, defined as time to the first MACE, death, date of point (D120) or date of last news, whichever occurred first.
Safety endpoints
Frequency of severe bleeding events (BARC classification>2) within 30 days, 90 days and 120 days following randomisation.
Frequency of bleeding events (any gravity) within 30 days, 90 days and 120 days following randomisation.
BARC, Bleeding Academic Research Consortium; CV, cardiovascular; ICU, intensive care unit; MACE, major adverse CV events.
Population
Eligibility criteria are detailed in box 2. Patients≥75 years, with a diagnosis of AP, hospitalised for at least 48 hours, with clinical signs onset<7 days and new radiology infiltrate will be included after informed consent.
Eligibility criteria
Inclusion criteria
Informed consent obtained from the patient or a relative/trusted person if the patient is unable to consent.
Age≥75 years.
Clinical diagnosis of acute pneumonia, presumed from bacterial or viral cause, with at least two of the following signs or symptoms: cough, purulent sputum, chest pain, dyspnoea/tachypnoea, temperature>37.8°C or<36°C and focal auscultatory findings.
New radiological infiltrate documented by X-ray, ultrasound or CT scan.
Onset of clinical signs<7 days.
Patient hospitalised for at least 48 hours.
Non-inclusion criteria
Ventilator-associated pneumonia.
Documented SARS CoV2 pneumonia.
Chronic swallowing disorders that compromise oral medication intake.
Patient with ≥3 episodes of aspiration pneumonia in the 12 months prior to inclusion.
Physician-assessed life expectancy<90 days.
Person not affiliated to a national social security scheme.
Patient under court protection.
Bleeding risk
Anticoagulant treatment (curative doses).
Antiplatelet therapy.
Steroidal or non-steroidal anti-inflammatory systemic treatment without proton pump inhibitor.
Dyspepsia or gastro-oesophageal reflux without proton pump inhibitor.
Active or recurrent peptic ulcer disease.
History of cerebrovascular haemorrhage.
History of digestive haemorrhage.
History of haemorrhage with haemoglobin>30 g/L requiring transfusion, vasoactive treatment or surgery.
Known hereditary or acquired coagulation disorder.
Thrombocytopenia (platelets<50 giga/L).
Contraindications to aspirin (preventive doses) or placebo at inclusion
Hypersensitivity to aspirin or to one of the excipients of the investigational drug or placebo.
History of asthma induced by salicylates or non-steroidal anti-inflammatory drugs.
Stage 5 chronic kidney disease (eGFR<15 mL/min).
Hepatic cirrhosis or severe hepatic insufficiency (PT<50%).
Severe uncontrolled heart failure.
Mastocytosis.
Persistent severe high blood pressure (systolic arterial pressure>180 mm Hg).
Methotrexate use>20 mg per week.
Prescription of anagrelide, probenecide, nicorandil and defibrotide.
eGFR, estimated glomerular filtration rate; PT, prothrombin time.
Study procedures
Screening, inclusion and randomisation
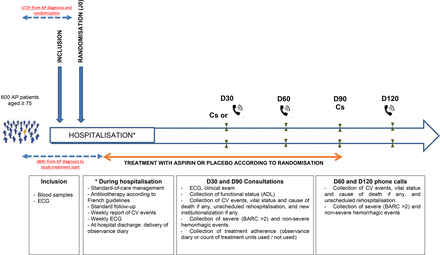
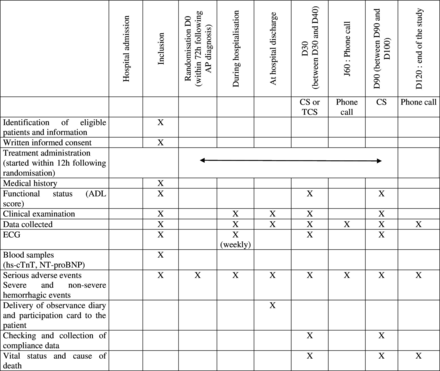
All patients with a diagnosis of AP will be systematically screened in each centre by investigators helped by a clinical research technician (CRT). This screening will start in the emergency department and will be performed in all medical departments receiving 75-year-old and above patients with AP (including geriatrics, infectious disease, pulmonology, internal medicine, intensive care departments). If the patient meets the eligibility criteria, the investigating physician will present the study to him/her or to his/her surrogate. The patient will be included after written consent (or consent of the surrogate if the patient is unable to receive and understand the information). An ECG and blood sample will be taken as soon as possible after inclusion (within 12 hours of inclusion) and before the first dose of treatment. Microbiological samples will be taken at the discretion of the clinician, in accordance with current guidelines (figure 1).24
Timing of data collection. ADL, activity of daily living; AP, acute pneumonia; BARC, Bleeding Academic Research Consortium; CV, cardiovascular.
Patients will be randomised within 72 hours following AP diagnosis to either the study group (aspirin) or the control group (placebo). Randomisation will be performed online by the investigator using the secure CleanWeb platform. Allocation will be based on a minimisation algorithm with a 1:1 ratio and an alea of 20%, and stratified on centre, age (<85/≥85), history of CV events (yes/no) and Pneumonia Severity Index (≤4/>4).25 Treatment (aspirin or placebo) should be started as soon as possible after randomisation, at most within 12 hours after randomisation, that is, within 84 hours following AP diagnosis.
Patients will receive 100 mg of aspirin or placebo orally (tablet), according to the randomisation arm, one daily (or intravenous during hospitalisation if the oral route is impossible) for 90 days.
Blinding process
The aspirin or placebo tablets will be packaged in blisters and cartons with 100 tablets. Each package will be numbered. If the intravenous route is required, an unblinded nurse will be required to prepare extemporaneous dose of 100 mg of aspirin or placebo. In the aspirin arm, the patient will be injected, after reconstitution, 100 mg of aspirin into a 100 mL NaCl 0.9% bag. In the placebo arm, the patient will receive 100 mL of NaCl 0.9%. The unblinded nurse will not participate in the patient care during the hospitalisation.
Blind may be lifted in case of a haemorrhagic event or in case of an accidental or intentional taking by a person other than the participant, only if unblinding may influence medical management of the acute situation, at the clinician’s discretion.
Follow-up during hospitalisation
Standard-of-care management and follow-up will be delivered according to French guidelines,24 including antibiotic systemic treatment, oxygen therapy, invasive and non-invasive ventilation if needed and prevention of thromboembolic disease. Site-of-care decision, as well as microbiological sampling, mode of radiological diagnostic confirmation, antibiotic choice and duration, will be at the treating clinician’s discretion. CV follow-up and monitoring during the hospital stay will include a weekly report of MACE, weekly ECG and collection of severe and non-severe haemorrhagic events. Study treatment will be administered daily in tablet form if the oral route is deemed feasible by the clinician, and intravenously if not (figure 2).
Study flow chart. ADL, activity of daily living; AP, acute pneumonia; CS, consultation; hs-cTNT, high-sensitivity cardiac troponin T test; NT-proBNP, N-terminal pro-brain natriuretic peptide; TCS, teleconsultation.
At hospital discharge, information will be provided to the general practitioner regarding his/her patients participation in a clinical trial, a diary to record treatment observance, as well as an individual study participation card will be provided to all patients, and study treatment will be dispensed (quantity required for 90 days). In the event of a severe swallowing disorder contraindicating oral intake at home, treatment will be suspended until oral intake is restored.
Follow-up after hospital discharge
Follow-up after hospital discharge will include
Consultation (or by default teleconsultation) at D30 with a collection of MACE, ADL score, vital status and cause of death if any, and unscheduled rehospitalisation, severe and non-severe haemorrhagic events and treatment adherence (drug monitoring booklet).
Phone call at D60 for collecting MACE, vital status, cause of death if any, unscheduled rehospitalisation, severe and non-severe haemorrhagic events and treatment adherence.
Consultation at D90, including ECG, clinical exam, collection of MACE, ADL score, vital status and cause of death if any, unscheduled rehospitalisation, new institutionalisation, severe and non-severe haemorrhagic events and treatment adherence.
The study will end at D120. The CRT will contact the patient, his/her support person or his/her general practitioner by phone and will search in hospital medical files to collect MACE, vital status, cause of death if any, number of days of unscheduled rehospitalisation and severe haemorrhagic events if any. Vital status will be collected for all included patients.
Treatment discontinuation
The criteria for temporary or permanent discontinuation of treatment are detailed in box 3. Regardless of the reason for discontinuation, all randomised patients will be followed up and efficacy and safety data will be collected until the end of the study (120 days after randomisation), with the exception of those who have withdrawn their consent to follow-up.
Treatment discontinuation criteria
Temporary interruption
(for the duration required by the medical situation)
Non-severe bleeding (BARC score≤2).
Perioperative setting.
New contraindication to aspirin (including perioperative setting).
New indication for prohibited drugs (antiplatelet agents (including aspirin, clopidogrel, prasugrel, ticagrelor, glycoprotein IIb/IIIa inhibitors), curative anticoagulation, systemic treatment with steroidal or non-steroidal anti-inflammatory drugs without co-prescription with proton pump inhibitors).
Inability to take oral medication after discharge.
Permanent discontinuation
Severe bleeding (BARC score>2).
Eligibility criteria not met (wrongly included patient).
Exclusive palliative care (ie, discontinuation of non-comfort-related therapies).
Patient consent withdrawal.
BARC, Bleeding Academic Research Consortium.
Discontinuation of treatment will not result in unblinding, unless this may influence medical management of the acute situation, at the clinician’s discretion (see ‘Blinding process’).
Data collection
The data will be entered directly by the investigator helped by a CRT into an electronic Case Report Form (e-CRF) specifically developed for this study using a Clinical Data Management System (CMDS; CleanWeb). All required information will be entered as and when it is obtained (at baseline and at each follow-up). Automatic checks for missing or inconsistent data will be integrated. These checks will follow the data management plan jointly defined by the coordinating centre in collaboration with the coordinating investigator. In case of missing or inconsistent data identification, requests for correction will be sent to participating centres via the CMDS. If corrections are necessary, they will be made by the CRT or by the investigator directly using CleanWeb.
The electronic system will ensure the traceability of every change made on the e-CRF.
A blind review of data will be performed regularly during the study and may result in additional queries. All queries should be resolved before the final database lock.
Any modifications to the protocol which may impact the conduct of the study, potential benefit of the patient or may affect patient safety, including changes in study objectives, study design, patient population, sample sizes, study procedures or significant administrative aspects, will require a formal amendment to the protocol. Such amendment will be approved by the Ethics Committee prior to implementation and notified to the health authorities in accordance with local regulations.
A data monitoring committee was not setup for this study as no interim analysis was planned. The data lock/unlock will be performed according to the procedure set up in the coordinating centre.
Sample size
The planned sample size of 500 patients analysable required (250×2) was estimated with an expected mortality of 25%14 26 and an expected reduction of 10% in 90-day mortality in the aspirin group (considered both probable19 22 and clinically relevant) with a two-sided alpha risk of 5% and power of 80%. To account for screening failures and consent withdrawals, 600 patients (300 per arm) will be included.
Statistical analysis
Population
The intention-to-treat (ITT) population will consist of all randomised patients who consented to participate in the study and will complete a modified ITT (mITT) analysis excluding patients who do not meet the inclusion criteria (or non-inclusion criteria).
Two per-protocol (PP) populations will be defined: one at D30 and one at D90. The PP population at D30 will exclude all subjects in the mITT population with a major deviation to treatment protocol (patients who did not receive the treatment allocated by their randomisation arm or non-treated patients or adherence<50% of the total dose at D30). The PP population at D90 will exclude all subjects in the mITT population with a major deviation to treatment protocol (patients who did not receive the treatment allocated by their randomisation arm or non-treated patients or adherence<50% of the total dose at D90).
Safety analyses will be performed on the safety population, which will comprise all randomised patients who consented to participate in the study and who have been given at least one administration of treatment.
Baseline characteristics
Patients’ characteristics at baseline will be described in terms of frequencies for categorical variables, and in terms of means (±SD) or medians (IQR) for continuous variables depending on their distribution.
Primary efficacy analysis
The main analysis will be conducted on an ITT basis. The effect of treatment of all-cause mortality at D90 will be assessed by logistic regression adjusted for minimisation variables in all randomised patients (including patients with intercurrent events according to the treatment policy strategy). The conclusion of the trial will only rely on this analysis.
This analysis will be completed by multivariable logistic regression, including minimisation variables and unbalanced baseline characteristics (at a threshold of p<5%), with respect to the model hypothesis. Results will be expressed using ORs and their 95% CIs. This latest analysis will be completed with an mITT and a PP analysis.
Secondary analyses
The treatment effect on qualitative endpoints will be assessed with a strategy similar to the one used in the main analysis (multivariate analysis with logistic regression).
The treatment effect on the length of first hospital stay will be assessed using a simple and multivariable (adjustment on stratification factors and unbalanced baseline characteristics if any) linear regression if model hypotheses are satisfied or non-parametric method (Mann-Whitney test) if they are not.
Overall survival will be calculated from randomisation to death or to point date (D120), whichever occurred first. MACE-free survival will be calculated from randomisation to MACE or to point date (D120), whichever occurred first. Patients who prematurely ended the study before observing death or MACE will be censored at the last assessment. Patients still alive and event-free will be censored on the date point (D120) or on their last assessment if occurring before D120. Time to endpoints will be summarised in each arm with the Kaplan-Meier method and compared between the arms with the log-rank test. This analysis will be completed by an analysis of the risk of death associated with the randomisation arm using the Cox model after adjustment for stratification and unbalanced baseline characteristics at the 5% threshold. For adjusted analysis, the number of factors introduced will respect a ratio of 10 events by estimated parameters.
Descriptive statistics will be used to summarise adverse events and compare the two groups.
Subgroup analysis
Subgroup analysis will be carried out to explore the effect of treatment on different subpopulations. Subgroup analyses will focus on age (<85/≥85, expected median age26), Pneumonia Severity Index (≤4/>4), hypersensitive troponin T (≤14 ng/L/>14 ng/L), N-terminal pro-brain natriuretic peptide (≤2000 pg/mL/>2000 pg/mL) and presence (yes/no) of a previous MACE (myocardial infarction, stroke, acute heart failure, atrial fibrillation, pulmonary embolism). For each subgroup analysis, the presence of an interaction between treatment and each of the variables studied on the endpoint under consideration will be tested. If the p of interaction is <0.10, a multivariate analysis adjusted for variables appearing unbalanced between subgroups will be performed. The level of significance is set to 0.05 for all analyses.
Ethics and dissemination
This trial was submitted to the Clinical Trials Information System (CTIS) provided by the European Medicines Agency for ethical and regulatory approval in France. The trial was approved by an independent ethics committee on 7 November 2024 (CPP Est 1, approval number: 2024-510811-32-00) and the French National Agency for the Safety of Medicines and Health Products and was authorised on 4 December 2024. The study will be conducted in accordance with the ethical principles of the Declaration of Helsinki and the recommendations of the Good Clinical Practices guidelines.
This trial was registered on Clinicaltrials.gov (NCT06774846) and on CTIS (EU CTIS number: 2024-510811-32-00).
The valid trial protocol at the time of submission is V.1.1, dated 5 November 2024.
The planning and conduct of this study are governed by the French law (law no. 2012-300 of 5 March 2012 relating to the research involving the human person as amended by order no. 2016-800 of 16 June 2016 and its implementing decrees) and European law (EU regulation 2017/745 relating to medical devices and EU regulation 536/2014 relating to clinical trials on medicinal products for human use).
Vulnerable participants will be included. Clinical studies in older frail patients are scarce, in part because informed consent is difficult to obtain due to delirium or permanent cognitive impairment. Some eligible patients, particularly those with severe AP, will not be able to receive and understand the information. However, the inclusion of such patients is necessary to ensure that the results can be extrapolated to the geriatric population as a whole.
If the patient is able to consent, he/she will be invited to participate in the study and will be asked to read and sign the consent. If the patient is unable to receive and understand the information, but a relative or trusted support person is present, the information will be given to the relative or trusted support person. The investigators will have to obtain the patient’s consent as soon as he or she has regained the capacity to consent. If the patient is under guardianship, consent will be obtained from his/her legal representative.
Data storage will be handled according to regulations.
The results of the study will be disseminated through conference presentations at national and international conferences and peer-reviewed manuscripts published in open-access journals.
Patient and public involvement
Patients and public will not be involved in the design, or conduct, or reporting, or dissemination plans of our research.
Discussion and implications
Older age is a major risk factor for both AP and CV events. Although mortality due to AP is particularly high in this age group, there are no specific interventional studies in the very old. Our project focuses on a large population of older patients hospitalised for AP, who are generally under-represented or absent from clinical trials. This geriatric population has specific prognostic features, particularly in relation to CV events, which severely affect long-term prognosis.27 No study has yet evaluated the efficacy of CV prevention with aspirin introduced as AP diagnosis in this specific population. More generally, there are no randomised double-blind trials evaluating the efficacy of aspirin in preventing all-cause mortality after AP.
If we can demonstrate that preventive doses of aspirin reduce mortality in patients hospitalised for AP, this will have a considerable impact on the management of AP. As this infection is one of the main causes of hospitalisation and death in older people, these results would be of public health interest. This frail population, often neglected by medical research, represents a growing proportion of the hospitalised population in industrialised countries.
Heart failure due to ischaemic heart disease and the functional consequences of stroke potentially preventable by aspirin are often responsible for the severe loss of independence and reduced quality of life. Despite recent advances in the management of stroke and myocardial infarction, curative treatment (thrombolysis, angioplasty) is still not widely used in older patients. Preventing CV events is therefore a public health priority, particularly in selected at-risk population.
This study has several limitations: first, because of increased bleeding risk under aspirin, patients at haemorrhagic risk will not be included, including those with a digestive bleeding history and those under anticoagulant or antiplatelet drugs at admission, which are particularly frequent in this old population. Second, CV events mostly occur at the early stage of AP, and thus delay in inclusion could decrease aspiration preventive efficiency.
Third, even though the non-inclusion criteria have deliberately limited their inclusion, some of the patients included (respiratory distress, swallowing problems, vigilance problems) will not be able to take the oral treatment. In such cases, the protocol provides for an intravenous relay during hospitalisation but not at home, resulting in treatment discontinuation for the time required by the clinical situation.
Unlike Oz et al, who focused their trial on CV prevention,22 we chose all-cause mortality as the primary endpoint. Indeed, we consider that improving overall survival remains the main issue in the management of AP, and not just the prevention of CV events, which are frequently underdiagnosed in this population. The strength of this study also lies in the potential generalisability of the results to a large population, given the broad inclusion criteria. In addition, the inclusions are multicentre and involve all the medical services concerned with the medical management of patients with AP.
We believe that this project will help improve the overall geriatric management of older patients by overcoming the usual duality between bacteria and antibiotic therapy in AP and by placing older patients at high risk of CV at the medical management centre. Primary prevention must remain a health priority in order to prevent both mortality and dependency in this frail population.
Ethics statements
Patient consent for publication
References
Footnotes
Collaborators ASPAPY study group: Mortada Adjim, Amal Aidoud, Thomas Brunet, Tomasz Chroboczek, Michele Collart, Matthieu Coulongeat, Guillaume Deschasse, Sylvain Diamantis, Gaëtan Gavazzi, Caroline Laborde, Sarah Lelarge, Nadège Lemarie, Lionel Piroth and Claire Roubaud-Baudron.
Contributors Study concept and design: AP, PM, MC and IF. Draft of the manuscript, primary responsibility for the final content and guarantor: AP. Statistical analysis: EK. Revision of the manuscript, read and approved the manuscript for final publication: all authors.
Funding This study is funded by the French Ministry of Health (Programme Hospitalier de Recherche Clinique—PHRC-20-0704). Trial sponsor: Centre Hospitalier Universitaire Dijon Bourgogne, Dijon, France.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; peer reviewed for ethical and funding approval prior to submission.