Article Text
Abstract
Objective There is limited evidence on the economic implications of assessing patients’ access to personalised treatments through Comprehensive Genomic Profiling (CGP) and Molecular Tumour Board (MTB), prompting the need to analyse their impact on the cost of the cancer diagnostic journey (from hospital admission to MTB evaluation) and accessibility to personalised therapies.
Design Retrospective observational cohort.
Setting Patients discussed from April 2020 to September 2021 by the institutional MTB operating at Fondazione IRCCS Istituto Nazionale Tumori of Milan, an Italian centre of excellence in oncology pertaining to the national health system.
Participants 676 patients focused on: non-small cell lung cancer (NSCLC), cholangiocarcinoma (CCA), pancreatic carcinoma (PC) and gastro-oesophageal carcinoma (GEC). We defined two different scenarios: (1) patients tested with small Next-Generation Sequencing (NGS) panels (≤60 biomarkers) vs (2) patients tested with comprehensive panels (>60 biomarkers).
Main outcomes and measures We measured (1) patients’ eligibility to personalised therapies based on genomic data obtained using targeted somatic NGS panels, (2) MTB cost and the overall diagnostic journey cost and (3) the cost to find a patient eligible to access personalised treatments.
Results Tumour profiling with comprehensive NGS panels improved patients’ eligibility to personalised therapies compared with small panels (NSCLC: 39% comprehensive panel vs 37% small panel; CCA: 43% vs 17%; PC: 35% vs 3%; GEC: 40% vs 0%). The overall diagnostic journey cost per patient was between 3.2K and 7.4K (NSCLC: 7.4K comprehensive panel vs 6.4K small panel; CCA: 4.9K vs 3.7K; PC: 5.8K vs 4.5K; GEC: 4.2K vs 3.2K). MTB discussion accounted for only 2–3% of the diagnostic journey cost per patient (around 113€/patient). The cost to find patient eligible for personalised treatments varied significantly according to panel size and tumour setting (NSCLC: 5K comprehensive panel vs 2.8K small panel; CCA: 4.4K vs 4.4K; PC: 5.5K vs 27K; GEC: 5.2K vs not measurable since none of the patients analysed with small NGS panels were eligible).
Conclusions and relevance MTB discussion of genomic data obtained with NGS comprehensive panels significantly increases patient eligibility to targeted therapies and optimise the cost to find a patient eligible to personalised treatments, mainly for CCA, PC and GEC patients.
- MOLECULAR BIOLOGY
- Health Services Accessibility
- Health Care Costs
Data availability statement
Data are available upon reasonable request. The data analyzed during the study are available from the corresponding author on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
The cohort consists of a large, ‘real world’, sample collection representative of the clinical practice of a referral cancer centre.
Given the retrospective nature of the study, information on long-term clinical outcomes or long-term treatments is lacking.
The current cost structure analysed in the study may change over time.
The Molecular Tumour Board cost was calculated based solely on hospital personnel expenses, without considering clinical outcome-based efficacy measures.
Introduction
Precision oncology is radically changing the treatment strategy for a growing number of oncological patients, resulting in substantial benefits in terms of clinical outcomes and disease prognosis.1 2
Next-Generation Sequencing (NGS) plays a fundamental role in precision oncology, allowing the simultaneous testing of a vast number of biomarkers and providing a huge amount of information to identify the most suitable treatment strategy for each patient.3–6 In addition, there is extensive evidence that introducing NGS as the standard testing approach for certain types of cancer, rather than single-gene testing approach, potentially leads to economic benefits for the National Health System (NHS).7 8 Moreover, given that the number of actionable genomic alterations and approved targeted therapies is steadily increasing, the implementation of Comprehensive Genomic Profiling (CGP) has become crucial to detect clinically relevant genomic alterations.2 9 10 Correctly interpreting the large amount of NGS data and translating these findings into clinical indications is challenging and requires different competences.11 Therefore, the introduction of MTBs is fundamental to select the most appropriate personalised treatment for each patient.12
MTBs are multidisciplinary teams composed of different healthcare professionals (eg, medical oncologist, geneticist, haematologist, pathologist, bioinformatician, molecular biologist, hospital pharmacist), which allow a comprehensive evaluation of the genetic data and patient characteristics, as well as the selection of the most appropriate treatment.11 13–15 A retrospective study conducted in the USA demonstrated that the combination of NGS and MTB allows for a significant reduction in drug costs related to inappropriate targeted therapy prescription.16 Moreover, the personalised treatment recommended by MTB differed from those indicated in the NGS vendor’s report in 45,6% of patients analysed.16
We and other groups provided evidence that MTBs not only play a role in recommending approved Standard of Care personalised treatments,10 but they also have the added value of enabling patients’ access to targeted therapies approved for other indications (off-label) or investigated in clinical trials (CTs).12 17 For these reasons, a process of implementation of MTBs is taking place in the context of national health systems.18
Our study, MTBsquare, focused on the MTB role in precision oncology. Even though NGS cost analyses have been previously carried out by different groups,7 8 there is still little evidence on the economic and organisational implications of MTBs (eg, MTB cost per patient and its relevance on the total patient journey cost).11
We aimed to measure the impact of combining NGS and MTB in terms of (1) relevance of MTB cost on the mean total diagnostic journey cost per patient, (2) eligibility to personalised treatments and (3) the cost to find a patient eligible to access targeted therapies according to different NGS testing strategies (small vs comprehensive NGS panels) and cancer settings—non-small cell lung cancer (NSCLC), cholangiocarcinoma (CCA), pancreatic carcinoma (PC) and gastro-oesophageal carcinoma (GEC). We focused on these tumour histology subtypes since they (1) present multiple actionable alterations; (2) have different European Society for Medical Oncology (ESMO) recommendations in terms of NGS testing at the time of the study (NGS recommended upfront in daily practice for NSCLC and CCA patients and limited to clinical research centres for PC and GEC patients)19 20; (3) are among the ten cancers with the highest mortality rate in the world1; and (4) are heterogeneous in terms of prevalence and incidence (online supplemental eTable 1).21
Supplemental material
Methods
MTBsquare, a retrospective observational study, was carried out in collaboration with Fondazione IRCCS Istituto Nazionale dei Tumori (INT) of Milan, pioneer and innovator in the implementation of MTB in Italy.10
Patient and public involvement
None.
Data collection
INT’s Ethical Committee approved the study with the protocol ‘code INT-277/20’ (21/12/2020); MTBsquare was performed in accordance with the Declaration of Helsinki. All the patients signed an informed consent to this observational prospective study at the time of specimen collection for NGS analysis. The study analysed the data of 676 oncological patients evaluated by the institutional molecular tumour board. Patient recruitment began in April 2020 and was completed in September 2021: 458 NSCLC, 65 CCA, 77 PC and 77 GEC patients. Additional information on patients’ population was reported in online supplemental eTable 2.
Data were collected exploiting different and complementary sources:
A structured database tracking pseudonymised patients’ evaluations by the institutional MTB, recording demographics, diagnosis, treatment line, NGS panels, eligibility for targeted therapies and recommended Active Pharmaceutical Ingredients (APIs) with regulatory approval status (AIFA, EMA, FDA). Targeted indications were classified as on-label or off-label. For off-label drugs, recruitment in Italian clinical trials was also recorded.
Interviews to HCPs (two oncologists, one geneticist, one pathologist, one bioinformatician, two biologists) to map and quantify patient diagnostic processes.
Open-access datasets containing rates of outpatient healthcare services in the Lombardia region22 and of surgical interventions in Italy.23 24
Literature to estimate the NGS annual maintenance equipment cost,25 the equipment payback period26 27 and overheads cost related to NGS testing.27
Along with the clinical-pathological and genomic data, we also analysed the diagnostic procedures to measure the impact of the MTB on the whole patient diagnostic journey, including all the activities prescribed by healthcare professionals (HCPs) from hospital admission to MTB discussion (online supplemental eFigure1–4). For this purpose, the overall patient diagnostic journey was divided into three main phases:
Phase 1: all the healthcare services before tumour genomic profiling to elaborate patient diagnosis, evaluate cancer stage and prognosis, determine if the patient could be eligible for surgery and treat the disease.
Phase 2: tumour genomic profiling carried out with NGS technology and other complementary ancillary tests (eg, HER2 for GEC).
Phase 3: discussion of the patient clinical case and tumour genomic profiling results by the MTB to select the most appropriate treatment strategy.
The impact of MTB was investigated according to different NGS testing scenarios:
Small NGS panel(s), where NGS panels including up to 60 biomarkers were used to test patients, which will be discussed in MTB. This scenario replicates one of the approaches adopted by multiple MTBs in Italy in the latest years.13 28
Comprehensive NGS panel, where patients evaluated by MTB were tested applying NGS panels larger than 60 biomarkers28 (Delibera Giunta Regionale 3 Giugno 2024 - n. XII/2442 Del “Molecular Tumour Board” Regionale Ed Individuazione Dei Servizi Di Medicina Di Laboratorio Specialistici per l’esecuzione Dei Test per La Profilazione Genomica Estesa Next Generation Sequencing (NGS) Ai Sensi Del d.m. Salute Del 30 2023, 2024).
Detailed data on panels used are reported in online supplemental data. This scenario represents the most appropriate precision oncology model, which would drive CT and off-label targeted therapies access.
Supplemental material
The study aimed to measure from the perspective of the national health system:
MTB cost and its relevance on the mean total diagnostic journey cost.
Patients’ eligibility to personalised treatments.
The cost to find a patient eligible to access personalised treatments.
Costs from the societal perspective were not investigated, including those for patients and caregivers, as the time required for NGS and MTB procedures is minimal (2–3 hours for discussing MTB recommendations with the referral oncologist, including time spent travelling to the hospital, completing administrative procedures, and any waiting times).
Regarding the first objective—MTB costs and their impact on the overall diagnostic pathway—we calculated the mean total diagnostic journey cost by cancer subtype, summing up the average cost of each phase.
To analyse Phase 1, as in the diagnostic path up to (but not excluding) the genomic profiling, all healthcare services that patients underwent from hospital admission to tumour genomic profiling were mapped, and the cost of each activity was summed considering its frequency of occurrence. A mean phase 1 cost was then calculated for each possible patient diagnostic journey within each cancer setting, as well as the percentage of patients for each scenario. The average phase 1 cost for each cancer type was finally determined by computing the weighted average of the cost for each possible diagnostic journey. Most healthcare services were reimbursed by the NHS, hence costs were extracted from the official fees. For the only healthcare service not included in the official outpatient fee, the costs were estimated based solely on personnel expenses, excluding consumables or overheads, as they were not relevant. Moreover, drug costs and expenses related to adverse events (AEs) management were excluded. Hospital personnel costs were estimated by multiplying the time required for each healthcare service by the hourly full hospital cost of the corresponding professional (eg, multidisciplinary team discussion to assess surgical eligibility).
For Phase 2, tumour genomic profiling, the cost analysis applied in this phase follows the approach described by Pruneri et al in their work on NGS in clinical practice,7 considering four cost components: personnel, consumables, equipment (purchase and maintenance) and overheads. The input data7 were reviewed and updated as needed (eg, average full hospital cost per professional, consumables kit costs).7 To calculate the equipment cost per patient, the annual volume of tumour genomic profiling tests performed using in-house NGS technology was estimated, assuming a monthly uniform test distribution (1.202 NGS panels/year). The annual equipment cost was then divided by the annual tests volume to obtain a fixed equipment cost per test.
Different NGS panels or their combination could be used for each cancer type. Therefore, a total cost per panel combination was calculated by summing the total cost of each panel used per patient, resulting in the total cost of the NGS testing strategy. To derive the average total cost per scenario for each cancer type, a weighted average was computed on the frequency of each testing strategy.
Finally, MTB costs (Phase 3) were estimated based on hospital personnel cost. The time spent per patient—before, during and after MTB—by each professional was converted into economic value using activity frequencies and the corresponding hourly hospital cost.
Phase 2 and 3 costs varied by scenario, since time to perform some activities (ie, to prepare MTB discussion) varied according to the testing strategy, and the cost of consumables differed by NGS panel type and outsourcing (Phase 2).
The impact of MTB costs on the overall diagnostic pathway was assessed by calculating the proportion of Phase 3 relative to the total diagnostic journey cost (Phase 1+Phase 2+Phase 3).
As for the study’ second objective, patient eligibility for personalised treatment was assessed by determining the prevalence of patients eligible for targeted therapies and the treatment category recommended by the MTB. Treatment categories—on-label, off-label and CTs—were classified based on the approval status of APIs by Regulatory Agencies (AIFA, EMA, FDA) using the prospectively annotated MTB database. In 14% of cases, the category of the recommended targeted therapy was updated during the observed period (ie, a shift from CT to on-label). In a small number of patients (2%), multiple targeted therapy recommendations were made by MTB, and the following was assumed in these cases:
When both on-label and CT therapies were recommended, the treatment category was classified as on-label, prioritising formally approved treatments.
When both off-label and CT therapies were recommended, the treatment category was classified as CT.
For the last project objective, the cost of identifying a patient eligible for personalised therapies was assessed, to compare costs and benefits of different precision oncology models for the NHS, as follows:
Costs focused on tumour genomic profiling (Phase 2) and MTB (Phase 3) costs, excluding expenditures related to all healthcare services before tumour genomic profiling (Phase 1), as they do not differ across diagnostic journey.
Benefits were measured as the number of patients eligible for targeted therapies following MTB evaluation, assuming that personalised treatment would improve patient prognosis compared with a non-targeted approach.11
The costs of NGS testing and MTB for all patients were divided by the number of eligible patients in the following scenarios:
Current clinical practice, where both small and comprehensive NGS panels are used (NSCLC: 95% small vs 5% comprehensive NGS panel; 18% vs 82% CCA, 52% vs 48% PC, 6% vs 94% GEC);
Small NGS panel, where all patients were hypothetically tested only with NGS panels ≤60 biomarkers.
Comprehensive NGS panels, where all patients were hypothetically tested with NGS panels larger biomarkers.
Additionally, the analysis considered patient eligibility for (i) all categories of targeted therapies (on-label, off-label, CTs), as per current MTB practice in Italy; and (ii) off-label and CTs personalised treatment, where MTB provides the most value in terms of enabling access to personalised treatments.
Results
The result section highlights the following outcomes: (1) MTB relevance on the mean total diagnostic journey cost, (2) patients’ eligibility to personalised treatments and (3) the cost to find a patient eligible to access personalised treatments.
MTB cost and its relevance on the mean total diagnostic journey cost
The total mean diagnostic journey cost per patient was higher for patients tested with comprehensive NGS panel (NSCLC: 6.425€/patient small vs 7.357€/patient comprehensive NGS panel; CCA: 3.695€/pt vs 4.904€/pt; PC: 4.509€/pt vs 5.774€/pt; GEC: 3.239€/pt vs 4.213€/pt) (figure 1). The most expensive phase of the patient diagnostic journey was Phase 1, which included all the healthcare services performed before tumour genomic profiling (from 50% to 85% of the overall diagnostic journey expenditure). MTB evaluation (Phase 3) cost was between 113€/patient (small NGS panel) and 118€/patient (comprehensive NGS panel), with a negligible impact (2–3%) on the mean total cost of the diagnostic pathway. The personnel time needed to evaluate a patient in MTB was 2.6–2.7 hours. In the Supplement, additional information about MTB resources utilisation, including time and costs by HCP (online supplemental eTable 3 and online supplemental eFigure 5); cost by activities performed pre, during and post MTB discussion (online supplemental eFigure 6) and Phase 2 costs (online supplemental eTable 4).
The mean total diagnostic journey cost per patient by cancer subtype and scenario. The average diagnostic journey cost per patient comparing small and comprehensive NGS panel approaches is shown for each cancer subtype, including the cost of activities before tumour genomic profiling (Phase 1), tumour genomic profiling (Phase 2) and MTB (Phase 3). CCA, cholangiocarcinoma; Compr, comprehensive NGS panel; GEC, gastro-oesophageal carcinoma; MTB, molecular tumour board; NSCLC, non-small-cell lung cancer; PC, pancreatic carcinoma.
Patients’ eligibility to personalised treatments
Overall, patient eligibility to targeted therapies was 35%, and it varied according to different cancer subtypes (37% NSCLC; 38% CCA; 18% PC; 38% GEC), as presented in figure 2. The use of comprehensive NGS panels significantly improved patients’ eligibility to personalised treatments compared with small NGS panels. As shown in figure 3, the benefits of comprehensive NGS panels increased from NSCLC to CCA, PC and GEC (NSCLC: 37% small panel vs 39% comprehensive NGS panel; CCA: 17% vs 43%; PC: 3% vs 35%; GEC: 0% vs 40%). NGS with small panels did not identify any actionable biomarker in the five GEC patients evaluated: in accordance with current guidelines,19 29 all these cases were evaluated for HER2 by immunohistochemistry, which did not detect any positive cases.
Patients’ eligibility to targeted therapies by cancer subtype. The percentage of patients eligible to targeted therapies is shown for each cancer subtype. CCA, cholangiocarcinoma; Compr, comprehensive NGS panel; GEC, gastro-oesophageal carcinoma; MTB, molecular tumour board; NSCLC, non-small-cell lung cancer; PC, pancreatic carcinoma.
Patients’ eligibility to targeted therapies by cancer subtype and scenario. The percentage of patients eligible for targeted therapies is shown for each cancer subtype, comparing patients tested with small and comprehensive NGS panels before MTB discussion. CCA, cholangiocarcinoma; Compr, comprehensive NGS panel; GEC, gastro-oesophageal carcinoma; MTB, molecular tumour board; NSCLC, non-small-cell lung cancer; PC, pancreatic carcinoma.
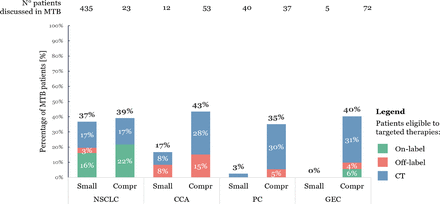
The categories of personalised treatments accessible for patients after MTB evaluation—on-label, off-label or CT—were assessed according to AIFA in patients eligible to targeted therapies. As shown in figure 4, most patients discussed in MTB were considered eligible to targeted therapies through CTs (NSCLC: 47%; CCA: 64%; PC: 86%; GEC: 76%). On-label personalised medicines were available for 44% of NSCLC patients and 14% of GEC patients. The eligibility to off-label targeted therapies was significant for CCA (36%), while it had minor relevance for other cancer subtypes (NSCLC: 9%; PC: 14%; GEC: 10%). Comprehensive NGS panels greatly enhanced the likelihood of accessing targeted therapies though CTs or off-label personalised medicines for CCA, PC and GEC patients (CTs: NSCLC: 17% small vs 17% comprehensive NGS panel; CCA: 8% vs 28%; PC: 3% vs 30%; GEC: 0% vs 31%. Off-label: NSCLC: 3% small vs 0% comprehensive NGS panel; CCA: 8% vs 15%; PC: 0% vs 5%; GC: 0% vs 4%), as reported in figure 5. In Supplement, the number of patients eligible for targeted therapies by API is reported for each cancer subtype (online supplemental eFigure 7).
Percentage of patients eligible to targeted therapy by drug category according to AIFA. The percentage distribution of patients evaluated by MTB and eligible to targeted therapies based on the different AIFA drug categories (on-label, off-label or CT) is shown for each cancer subtype. AIFA, Italian Medicines Agency; CCA, cholangiocarcinoma; CT, clinical trial; GEC, gastro-oesophageal carcinoma; NSCLC, non-small-cell lung cancer; PC, pancreatic carcinoma.
Percentage of patients eligible to targeted therapy by drug category according to AIFA by scenario. The percentage distribution of patients evaluated by MTB and eligible to targeted therapies based on the different AIFA drug categories (on-label, off-label or CT) is shown for each cancer subtype and scenario, comparing patients tested with small and comprehensive NGS panels. AIFA, Italian Medicines Agency; CCA, cholangiocarcinoma; CT, clinical trial; GEC, gastro-oesophageal carcinoma; NSCLC, non-small-cell lung cancer; PC, pancreatic carcinoma.
The percentage of patients eligible for on-label personalised treatment increased from AIFA to EMA and FDA (online supplemental eFigure 8).
The cost to find a patient eligible to access personalised treatments
The cost to find a patient eligible to personalised treatments for the National Health Service significantly decreased using comprehensive NGS panels in the setting of PC and GEC patients (NSCLC: 2,8K€ small vs 5,0K€ comprehensive NGS panel, CCA: 4,4K€ vs 4,4K€, PC: 27,3K€ vs 5,5K€, GEC: not measurable vs 5,2K€), as a result of a significant increase in the prevalence. of patients eligible for personalised medicines (NSCLC: 168 small vs 179 comprehensive NGS panel; CCA: 11 vs 28; PC: 2 vs 27; GEC: 0 vs 31). It was not possible to calculate the cost to find a patient eligible to access targeted therapies for GEC patients tested with small NGS panels, since none of them was eligible in the investigated sample. In the case of CCA patients, the cost to find a patient eligible for personalised treatment did not change across scenarios, even if the number of eligible patients increased when all the patients were tested by comprehensive NGS panels (+13% comprehensive NGS panels vs current clinical practice (28pt vs 25pt), + 160% comprehensive NGS panels vs small NGS panels (28pt vs 11pt)). Focusing on NSCLC, the difference between current clinical practice (95% patients tested with small and 5% with comprehensive NGS panels) and 100% small NGS panels scenario was around 100€ per patient. The comparison of the cost to find a patient eligible to access personalised treatments by cancer subtype and by scenario is summarised in table 1. The data on the cost to find a patient eligible to access targeted therapies were also confirmed for off-label and CT targeted therapies: the cost to find a patient eligible was optimised testing all patients with comprehensive NGS panels in case of CCA, PC and GEC (online supplemental eTable 5).
The cost to find a patient eligible to access personalised treatment in different scenarios (current clinical practice, small NGS panel, comprehensive NGS panel)
Discussion
MTBsquare is the first study to estimate the impact of different precision oncology models on costs and access to targeted therapies. Although the analysis was carried out in an Italian institution, the model generated can be extended to most of the national health systems.
The overall eligibility to personalised treatment by cancer subtype was between 37% and 38%, except for PC (18%). This is in line with data previously reported by different groups,17 30 including our own, with PC notoriously known as an ‘untargetable’ malignancy due to the low frequency of actionable alterations, with only partial advantages from late therapies mainly associated with germline alterations.31 We also found that the combination of CGP and MTB significantly enhanced patients’ accessibility to targeted therapies, especially to off-label and CTs treatments for CCA, PC and GEC patients (+26–34% cases eligible to off-label or CT targeted therapies). The advantages of this approach were confirmed by the ROME Trial, a wide real-world study involving 41 oncological centres in Italy, where 34% of patients (483/1,319) received a targeted therapy recommendation following comprehensive NGS analysis and MTB discussion.32 Along this line, the RATIONAL study supported the clinical advantages in adopting CGP data for MTB discussion with a higher rate of actionable genomic alteration in particular ESCAT scale tier II and III, which are mostly actionable with CTs and off-label therapies.9
Undoubtedly, the use of customised small panels investigating the biomarkers for which on-label drugs are available might significantly increase the chance to select patients for personalised therapies, for example, in virtue of faster analyses or less demanding computational efforts. On the other hand, customising different panels for each tumour cancer subtype would be costly and time-consuming and would require significant additional workload (and adequate cohorts) for internal validation. Furthermore, given the steady incorporation of new biomarkers in clinical practice, this would compel frequent adaptation of the customisation.
In the management of oncological patients, the therapeutic approach should not be limited to the use of on-label drugs but should necessarily extend to the rational and scientifically supported utilisation of off-label treatments, as well as to the access to targeted CTs. This paradigm reflects the growing need for precision medicine, which, through a synergistic integration of emerging evidence, advanced genomic profiling and multidisciplinary assessments, allows optimising therapeutic efficacy in the context of real-world clinical practice. Consequently, this approach inherently drives the preference for large DNA/RNA sequencing panels over small panels, exploiting personalised therapeutic opportunities. Other reasons prompt sustaining the advantages described by the eligibility improvement, particularly for PC and GEC. Among these, copy number variations (CNVs) are emerging as a promising biomarker for treatment stratification, particularly in cancer settings in which on-label treatments are scarce. This dynamic scenario underscores the necessity for more comprehensive genomic analysis beyond standard sequencing panels, to achieve a more thorough molecular dissection capable of capturing clinically relevant CNVs.
The average MTB cost was marginal compared with the total diagnostic journey cost per patient (113–118€/patient), considering personnel costs. This result was in line with the study of Arnaud et al (120€/patient) conducted in France in 2017, confirming the negligible impact of MTB introduction on healthcare budget. Furthermore, the weight of MTB cost might be even lower when considering the cost of healthcare services after MTB evaluation or when including the cost of drugs and management of AEs (0.03% of the total cost).33
To the best of our knowledge, the cost to find a patient eligible to access personalised medicines was not investigated in other studies. Focusing on differential costs for the patient diagnostic journey in the precision oncology setting (tumour genomic profiling and MTB), the cost to find a patient eligible to access targeted therapies was optimised when adopting comprehensive NGS panels as testing strategy for all cases in CCA, PC and GEC patients. As regards NSCLC, the current mix of testing approaches defined by HPCs (95% small and 5% comprehensive NGS panels) might represent the proper model to guarantee both clinical effectiveness and an optimised resources allocation. A key factor influencing this result might be the higher number of approved targeted therapies for NSCLC patients compared with other cancer settings. These results might vary in the future since they are mainly influenced by NGS expenses and biomarker actionability. In particular, the cost to find a patient eligible to access personalised medicines would decrease in case of a lower NGS cost34 35 and a higher number of actionable biomarkers,36 boosting precision oncology extensive implementation. Nevertheless, it is important to implement CGP and MTB only in hospitals with qualified personnel, adequate infrastructures and high testing volumes, to leverage on economies of scale and scope and avoid wasting resources.
Our study based on real-world data added a piece of literature to define policy implications of different precision oncology models. However, our study has a number of potential flaws, since it was focused on a single Italian centre of excellence in oncology, reported current cost structure that might change in the future, and MTB cost was calculated considering only hospital personnel cost and did not include any efficacy measure based on clinical outcomes.
Combining NGS and MTB may have further benefits that were not estimated in the study: (1) significantly improving clinical outcomes and patient prognosis,17 37 (2) avoiding clinical and financial toxicities related to the improper therapy prescription,16 (3) improving patients and caregivers QoL38 39 and (4) finding potential pathogenic germline variants, which can lead to preventive measures and early diagnosis of cancer.9
Even though the increasing relevance of precision medicine for the shaping of the future of oncology39 and the proved value of CGP+MTB in enabling precision oncology implementation,9 32 there are several organisational and economic barriers to unlock equal and sustainable access to personalised treatments. To remove these barriers, it is fundamental (1) to improve national or regional laboratory, oncological and MTB networks40; (2) to harmonise tumour genomic profiling and MTB implementation approaches17 41 42; (3) to allocate resources for NGS and MTB (eg, establishing a national fee for MTB); and (4) to increase the number of personalised treatments in oncology. Furthermore, few eligible oncological patients with an actionable genomic variant actually receive personalised treatment (8,9/9,6–11,7%), as highlighted in two recent publications.9 10 The main reasons are (1) the difficulties in accessing off-label treatments, (2) few available CTs and (3) the poor patient clinical conditions.9 Marchetti et al proposed a new possible model called sub iudice procedure to speed up and facilitate oncological patients’ reimbursed access to personalised treatments recommended by MTB after extensive NGS profiling.41 Nowadays, the costs of approved targeted therapies are mostly reimbursed by the NHS, while pharmaceutical companies pay for CT drugs.43 In case of off-label therapies, approved for other indications, costs are charged to different actors according to the specific Italian regulation: NHS whether 648/96 Law is applicable, pharmaceutical companies if patients are included in compassionate use programmes,44 the Italian Medicines Agency if AIFA national fund (326/2003 Law) is exploited or to the patient if the abovementioned options are not viable.45 The new sub-judice procedure suggests that pharmaceutical companies provide as a first step free off-label drugs to patients who do not have other approved better therapeutic options and generate evidence by recording patients’ clinical data through non-profit CTs or observational studies for Market Access (MA) reasons. Then, when enough information is gathered, pharmaceutical companies might use collected RWD to apply for pricing and reimbursement (P&R) conditions for the potential new indication of the personalised anticancer drug.41
In this such evolving, complex and innovative scenario, affordable and structured real-world evidence (RWE) on the overall precision oncology model (NGS, MTB and treatment through targeted therapies), tracking both clinical and economic data, is fundamental to allow decision-makers to take informed decisions.41 46 Ideally, RWD should be collected in a standardised platform accessible from all clinicians involved in tumour genomic profiling, MTB and targeted therapies monitoring.41 46
Conclusions
MTB has a negligible cost on oncological patients’ diagnostic pathway compared with the impressive benefits as accessibility to innovative personalised medicines and prescription of the most appropriate drug to the patient. Therefore, MTB should have a crucial role in the precision oncology setting. Moreover, combining CGP and MTB significantly enhances patients’ eligibility to off-label and CTs targeted therapies and optimise the cost to find a patient eligible to personalised medicines for CCA, PC and GEC. To ensure both equal access to precision oncology for all patients and effective use of available resources, decision-makers must regulate MTB and CGP access, costs and organisational structure, based on RWE and considering the impact of different precision oncology approaches as costs and benefits.
Data availability statement
Data are available upon reasonable request. The data analyzed during the study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by INT’s Ethical Committee with the protocol 'code INT-277/20' (21/12/2020). Participants gave informed consent to participate in the study before taking part.
References
Footnotes
VDM, LA and EC are joint first authors.
Contributors Conceived and designed the research approach: VDM, ABa, AV, LA, GG, MF, JMF, GP and CJ. Provided and validated the input data: EC, AV, LA, MD, FP, ET, AP, DL, ABu, IC, MN, CP, CV, SM, FdB and GP. Designed the analytical model and analysed the data: VDM, VLRA, ABa and CJ. Interpretation of the analysis: VDM, VLRA, ABa, AV, LA, GP and CJ. Wrote the first draft: VDM, VLRA, ABa, AV, LA, GP and CJ. Guarantor: LA. All authors interpreted the data and commented on the first draft. All authors revised the first draft. All authors agreed with the final version. GP and CJ are joint last authors.
Funding This study was unconditionally sponsored by Roche S.p.A, which supported study design, analysis and interpretation of the data and report writing, and was funded by Ricerca Corrente, Ministero della Salute to GP.
Competing interests LA had a consulting role for Roche. M. Niger received speaker honorarium from Accademia della Medicina and Incyte; honoraria for editorial collaboration from Sandoz, Medpoint SRL, Incyte, AstraZeneca and Servier; consultant honoraria from EMD Serono, Basilea Pharmaceutica, Incyte, MSD Italia, Servier, Astrazeneca and Taiho Pharmaceutical; travel expenses from AstraZeneca. CP received honorarium from AstraZeneca, Roche, MSD, Bristol Myers Squibb; travel accommodation from AstraZeneca, Roche, MSD, Janssen, Sanofi; compensations for Advisory Board from AstraZeneca, Roche, MSD, Janssen, Sanofi and she was Principal Investigator in clinical trials of Janssen, Pfizer, Eli Lilly, Spectrum Pharmaceuticals, Roche, MSD, Bristol Myers Squibb, AstraZeneca, Daiichi Sankyo. GG and MF are employees of Roche S.p.A.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.