Article Text
Abstract
Introduction Approximately 70% of patients with stroke experience varying degrees of cognitive impairment, which imposes a substantial direct and indirect socioeconomic burden. Previous studies have shown that scalp acupuncture (SA) or repetitive transcranial magnetic stimulation (rTMS) in combination with other therapies is effective for poststroke cognitive impairment (PSCI). Limited by interstudy heterogeneity and the limited number of included studies, there is insufficient evidence of the efficacy of rTMS in combination with SA in treating PSCI. Therefore, this protocol aims to investigate the effectiveness of rTMS in conjunction with SA for patients with PSCI through a comprehensive meta-analysis.
Methods and analysis This study will undertake a comprehensive search across nine distinct databases (Web of Science, Embase, Cochrane Library, PubMed, China National Knowledge Infrastructure, Wanfang Data, China Science and Technology Journal Database, China Biology Medicine and SCOPUS). The primary outcome will encompass the Montreal Cognitive Assessment and the Mini-Mental State Examination. The secondary outcomes are the modified Barthel Index, the Rivermead Behavioral Memory Test and the Digit Span Test. The bias risk assessment tool from the Cochrane Handbook for Systematic Reviews of Interventions will be used to evaluate bias risk, and the GRADE will be applied to gauge the quality of evidence. Furthermore, we plan to perform an analysis of subgroups to investigate the heterogeneity, employ the leave-one-out approach for sensitivity evaluation and use funnel plots and Egger’s test to determine publication bias, respectively.
Ethics and dissemination Ethical approval is not required in systematic review and meta-analysis. The review will be published in a peer-reviewed journal.
PROSPERO registration number CRD42024571762.
- Transcranial Magnetic Stimulation
- Acupuncture
- Stroke
- Cognition
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
The study will incorporate clinical randomised controlled trials and observational trials to comprehensively investigate the effectiveness and even the superiority of repetitive transcranial magnetic stimulation combined with scalp acupuncture.
Qualitative (funnel plot) and quantitative (Egger’s test) methods will be used to assess this study’s potential publication bias and improve the robustness of the meta-analysis results.
The methodological rigour is enhanced through researchers’ independent, individual contributions to both data collection and the assessment of bias risks.
The varying severity of stroke in patients, non-uniform cognitive assessment tools and partial reliance on subjective outcome measures may lead to heterogeneity.
Inconsistencies in intervention protocols (eg, stimulation parameters, treatment frequency) and variable follow-up timelines could impact the reliability and generalisability of the results.
Introduction
Poststroke cognitive impairment (PSCI) frequently occurs as a complication in patients with stroke. It involves a decline in cognitive abilities such as visuospatial skills, naming, focus, language, delayed memory and orientation, reaching diagnostic criteria within 6 months following a stroke.1 Approximately 70% of patients who had a stroke experience varying degrees of cognitive impairment,2 which impacts the risk of recurrent stroke and contributes to increased morbidity and mortality rates.3 4 Moreover, PSCI significantly affects motor function5 and sleep,6 reduces patients’ quality of life,7 prolongs their hospital stays8 and finally imposes a substantial direct and indirect socioeconomic burden.3 In recognition of this, assessment and treatment of PSCI should be a top research priority in stroke, as it holds the key to enhancing stroke outcomes.9 Unfortunately, cognitive decline has not been brought to high attention, and there is insufficient evidence to reach a consensus on specific methods for cognitive rehabilitation after stroke.10
Currently, the commonly used clinical treatments of PSCI are drug therapy and cognitive rehabilitation training. Drug therapy can promote local blood supply, accelerate brain tissue energy metabolism and improve the cognitive function of patients.11–13 However, it has a limited therapeutic effect due to its side effects.14 15 Furthermore, there is currently no universally recognised specific drug for treating PSCI or dementia.2 On the other hand, cognitive rehabilitation training requires active participation from patients, which can be challenging and lead to low compliance rates, ultimately hindering optimal results.16 Some scholars have suggested that compared with the traditional ‘bottom-up’ rehabilitation approach, the ‘top-down’ neuromodulation technique may offer superior therapeutic effects.17 Neuromodulation technology is potentially one of the most effective rehabilitation treatments for motor recovery after stroke, such as repetitive transcranial magnetic stimulation (rTMS) and scalp acupuncture (SA).18
The non-intrusive method of rTMS creates a potent magnetic field via a robust coil, seamlessly penetrates the cerebral cortex through the skull and non-invasively stimulates the affected brain region, modulating brain activity and enhancing patient performance by influencing cortical excitability.19 Previous meta-analyses have demonstrated the effectiveness of rTMS in treating cognitive impairment after stroke.20 21 Furthermore, the network meta-analysis conducted by Yang et al in 2024 incorporated 22 randomised controlled trials (RCTs) focusing on non-pharmacological interventions for PSCI. Notably, rTMS emerged as the most promising therapy among the 14 treatment modalities, demonstrating the most significant efficacy in improving patients’ cognitive functions.22 This finding underscores rTMS as one of the most promising treatment options for PSCI. Interestingly, in a meta-analysis, Duan et al found that the combination of rTMS with other therapies was more effective in treating patients who had a stroke than using rTMS alone, and they suggested that it should be considered as one of the options for patients in clinical treatment.23
SA is a therapeutic approach that involves stimulating specific areas on the scalp with needles to treat various medical conditions. It boasts advantages such as low-cost effectiveness and high social benefits. Numerous clinical studies have confirmed its role in treating patients with stroke.24–28 Jiao et al proposed that rTMS combined with SA can effectively enhance patients’ cognitive function, and its therapeutic effect is independent of the intervention duration.29 A meta-analysis conducted by Li and colleagues further found that the combination of SA with eight other therapies, including rTMS, significantly improved cognitive function in patients with PSCI.30 It markedly boosts the levels of nerve growth factor, brain-derived neurotrophic factor and growth-related protein near cerebral infarction compared with single therapy, thereby providing better repair for neurological damage caused by strokes.31 It can also better regulate poststroke brain plasticity, strengthen functional brain connectivity32 33 and improve patients’ cognitive function.29
However, due to certain heterogeneities among the studies and the limited number of RCTs, there remains a lack of sufficient evidence to prove the effectiveness and superiority of rTMS in conjunction with SA. Furthermore, the efficacy of rTMS is closely related to its stimulation frequency. Additionally, since cognitive function comprises various cognitive dimensions, it remains unclear whether the combined intervention of rTMS and SA elicits distinct effects across various cognitive function domains. Therefore, we plan to conduct a comprehensive review and meta-analysis of clinical research investigating the effectiveness of rTMS in conjunction with SA in patients with PSCI to provide available evidence for combination therapy in PSCI.
In order to improve the usability and accessibility of systematic reviews, many experts have called for developing a protocol before conducting a systematic review.34 Formulating a protocol constitutes a pivotal component of the review process. It serves as a detailed planning document prepared in advance of initiating the review. Its significance manifests in several ways. First, it requires the team to specify the analysis framework in advance, helping to identify potential problems and reducing arbitrary decision-making in the implementation phase. Second, by making the protocol public, it enables readers to compare the plan with the final results, effectively identifying selective reporting bias and thus assessing the validity of the synthesis methodology and the reliability of the results. Third, disclosing the protocol avoids duplication of studies and allows peers to preassess the methodological validity, improving research efficiency. Consequently, we have developed this research protocol before conducting the systematic review. The distinct objectives of this protocol are outlined as follows:
Objective 1: Does the combination of rTMS and SA prove efficient in treating PSCI?
Objective 2: Would the effects of low-frequency rTMS combined with SA be identical to those of high-frequency rTMS combined with SA on PSCI?
Objective 3: Does the combined therapy of rTMS and SA exhibit varying efficacy across different cognitive dimensions in patients with PSCI?
Methods
This research constitutes a meticulously crafted protocol for a comprehensive systematic review and meta-analysis, aimed at elucidating the impact of rTMS in conjunction with SA on PSCI, which has been registered in PROSPERO (ID: CRD42024571762).
Criteria for selecting eligible studies
The articles included in this meta-analysis will undergo a rigorous examination of their selection criteria using the Participants, Intervention, Comparison and Outcome (PICO) framework as a structured approach.
Types of research
Studies that meet all of the following criteria will be included: (1) clinical study, (2) published in either English or Chinese and (3) registered before July 2024.
Studies that adhere to any of the following exclusion criteria will be omitted: (1) duplicated publication, (2) unavailable data studies, (3) basic experimental studies, (4) conference publication, (5) editorial materials and (6) retracted publication.
Types of participants
Inclusion criteria: (1) the inclusion criteria for participants required a diagnosis of stroke based on standardised diagnostic criteria, and confirmation of their first stroke event through CT or MRI, (2) confirmed cognitive impairment as assessed by specialised cognitive function scales, (3) over 18 years old and (4) patient was conscious with clear mental status, and vital signs remained stable.
Exclusion criteria: (1) history of other neurological disorders and (2) any mental illnesses that may potentially affect the test results.
Types of intervention
Patients in the trial group received active rTMS in conjunction with SA, with any stimulation parameters deemed eligible for the study.
Types of comparison
We plan to set up three control groups to progressively ascertain the efficacy of rTMS combined with SA. The first control will briefly analyse the therapeutic effects of rTMS+SA. Patients in the control group received conventional treatment. The second control aims to further determine whether the efficacy of rTMS+SA is superior to that of single interventions. Patients in the control group received either active rTMS alone or SA alone. The third control will be to exclude placebo effects and to analyse the efficacy of rTMS+SA. Patients in the control group received either active rTMS+sham SA or sham rTMS+active SA.
Types of outcomes
Primary outcomes will include the Montreal Cognitive Assessment and the Mini-Mental State Examination.35 36 The modified Barthel Index, serving as a secondary outcome, is employed to assess the quality of daily life.37 In addition, we will also incorporate the Rivermead Behavioral Memory Test to assess memory function and the Digit Span Test to evaluate attentional function, respectively.
Data search and strategy
This study will conduct a comprehensive search across nine databases from inception to July 2024, including Web of Science, Embase, Cochrane Library, PubMed, China National Knowledge Infrastructure, Wanfang Data, China Science and Technology Journal Database, China Biology Medicine and SCOPUS. Furthermore, we conducted a thorough examination of the reference lists sourced from recent reviews and diverse other materials to identify pertinent original articles.
Next, our search methodology will adhere to the PICO framework, incorporating key terms such as ‘stroke’, ‘transcranial magnetic stimulation’, ‘acupuncture’ and ‘cognition’. To ensure comprehensiveness, we will perform exhaustive searches across all relevant fields within each database using both Medical Subject Headings and free-text terms. The specific search strategy employed for PubMed is provided in online supplemental table S1.
Supplemental material
Data collection
Study selection
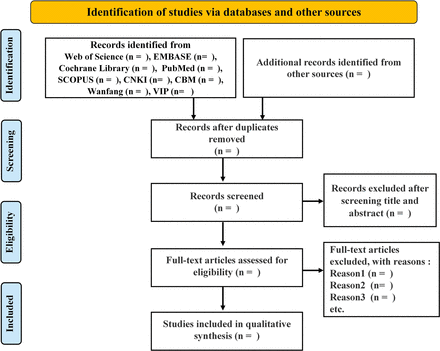
The entire process of literature screening will be conducted using NoteExpress V.3.5.0 software. The literature screening will comprise two rounds, with each round employing a double-screening mode by two authors. Specifically, two authors independently review and assess each article to determine its inclusion. When there is a conflict, seeking a third author’s viewpoint is the approach taken. During the first round, two authors (RZ and SC) will independently review the titles, abstracts and keywords of the articles to make an initial assessment. Studies that meet the preliminary criteria subsequently undergo a secondary screening phase. In the second round, the two authors (RZ and SC) will independently review the full texts of the articles and include eligible articles in the final meta-analysis. The whole process is presented in figure 1.
Flow diagram.
Data extraction
The data extraction process will adopt a double-entry method, whereby two evaluators will each independently enter the data into a predesigned, advanced Excel file. Subsequently, a final verification will be conducted to confirm the definitive version, effectively minimising the occurrence of errors. All eligible studies will be used for the extraction of data and information. We will gather the following details: study characteristics, trial design, general demographics of the participants, intervention methods for each group, parameters of rTMS, treatment duration for each group and outcome indicators both before and after the intervention. More details can be found in online supplemental table S2. In situations where a study presents numerous intervention alternatives, our consistent approach will be the χ2 comparability principle, choosing the two datasets that show minimal diversity in our analysis.
Assessment of bias risk
The assessment of bias risk is going to employ a double-assessment model, involving two researchers (RZ and SC) who independently evaluate the risk of bias in the included studies. This will be followed by a cross-checking process to enhance the quality of the assessment. If there are discrepancies between the two researchers’ opinions, a third reviewer will be consulted to make the final decision. The assessment tool used is the bias risk tool from the Cochrane Handbook for Systematic Reviews of Interventions, which categorises the risk of bias into three levels: ‘low risk of bias (+)’, ‘unclear (?)’ or ‘high risk of bias (−)’.38
Statistical analysis
Solution of missing data
Generally, we will contact the original authors through email or telephone to solicit any missing or incomplete data first. If this endeavour is unsuccessful and the incompleteness poses a potential risk of bias, we will then exclude those studies from our analysis, ensuring the integrity and reliability of our research findings.
Meta-analysis
When sufficient data are available to assess outcomes, and the studies demonstrate comparability in terms of design, methodology and interventions, a meta-analysis using STATA V.17 software will be undertaken to derive estimates of the combined effects. In the absence of such data, a descriptive analysis will be performed. For dichotomous variables, our analysis will focus on ORs with accompanying 95% CIs. Meanwhile, continuous variables will be presented in the form of mean differences with 95% CIs. The choice between a fixed-effects model (I²<50%) and a random-effects model (I²≥50%) will be dictated by the level of heterogeneity observed.39 If studies exhibit excessive heterogeneity, rendering them unsuitable for pooling, descriptive analyses will be conducted as an alternative.
Subgroup analysis
When sufficient data are available, we will conduct a subgroup analysis to further explore the effects of combined interventions on PSCI. A comparison of different frequencies of rTMS combined with SA against the control groups will be conducted, specifically examining the subgroups of low-frequency rTMS combined with SA versus the control group and high-frequency rTMS combined with SA versus the control group.
Sensitivity analysis
For enhancing the precision and authenticity of the primary outcome, we will perform a sensitivity analysis using the leave-one-out method. This rigorous process involves systematically eliminating specific studies, including those with small sample sizes, suboptimal quality or notable heterogeneity, to evaluate their individual influence on the aggregated findings.
Publication biases
Funnel plots will be used to evaluate the potential reporting bias within the included literatures. Nonetheless, acknowledging the subjective nature of funnel plots and the lack of objective numerical benchmarks, we will supplement this analysis with Egger’s regression as an additional quantitative tool to rigorously assess the publication biases.
Evidence quality
To thoroughly assess the quality of the studies included in this research, we will use the GRADE software, which categorises the quality into four levels: very low, low, moderate and high.40 This approach significantly reduces subjectivity and errors in the evaluation process and provides an overall assessment of the quality of the included studies. Consequently, it offers more comprehensive and objective evidence to support the conclusions of the meta-analysis.
Patient and public involvement
Our research is founded solely on published data, thereby excluding the direct involvement of patients or the general public in the design, execution, reporting and dissemination strategies of this study.
Ethics and dissemination
Ethical approval is not required in systematic review and meta-analysis. The review will be published in a peer-reviewed journal.
Ethics statements
Patient consent for publication
Footnotes
Contributors Formulation: HX, ML, LP. Data management: HX, RZ, SC. Methodology: HX, JJ, BH. Supervision: ML, LP. Project administration: HX, RZ, SC. Initial draft: HX. Revision and proofreading: HX, ML, LP. Guarantor: HX.
Funding This work was supported by the National Natural Science Foundation of China (No 82174521), the Projects of Science and Technology Innovation Plan in Hunan Province (No 2024JK2132, No 2024RC1061), the Changsha Municipal Science and Technology Plan Project (No kq1801184), the State Administration of Traditional Chinese Medicine 2022 Youth Qihuang Scholars Training Program (National Letter of Traditional Chinese Medicine Education (2022) No 256), the Hunan Provincial Graduate Joint Cultivation Base for Acupuncture-Moxibustion and Tuina of Hunan University of Chinese Medicine ((2022) No 357, Hunan Provincial Department of Education Notice), the Acupuncture Bioinformation and Smart Wellness Innovation and Entrepreneurship Education Center of Hunan University of Chinese Medicine ((2021) No 356, Hunan Provincial Department of Education Notice) and the Hunan Provincial Modern Industrial College–'Xiang'ai (Hunan Mugwort) Health Modern Industry College' of Hunan University of Chinese Medicine (approved and documented as Xiangjiaotong (2023) No 379).
Disclaimer The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.