Article Text
Abstract
Purpose We are conducting a longitudinal cohort study—the Community Burden of Acute Respiratory Infections in Shanghai—to assess age-stratified incidence, healthcare utilisation and risk factors of influenza virus, respiratory syncytial virus (RSV) and SARS-CoV-2 associated acute respiratory infections (ARIs) in Shanghai, China.
Participants Study participants were enrolled by family doctors in all 47 community health services centres in Pudong New Area District, Shanghai, China. All permanent residents 6 months and older living in Pudong for at least 6 months were eligible for enrolment; residents who planned to leave Pudong for more than 1 month in the first study year were excluded. During enrolment, study staff conducted baseline assessments of sociodemographics, underlying medical conditions, vaccination history and household and self-rated health status. Study participants are being followed for ARIs for 3 years. Nasopharyngeal and oropharyngeal swab specimens are being obtained from suspected ARI cases. Influenza virus, RSV, SARS-CoV-2 and other respiratory pathogens are tested for by multiplex respiratory pathogen real-time quantitative PCR assays. Illness courses and clinical recoveries of ARI cases are assessed through weekly contact with ARI cases for 28 days post ascertainment.
Findings to date Between 14 October 2024 and 22 November 2024, we enrolled 5387 community residents into the cohort, including 233 children aged from 6 months to 2 years, 278 preschool children aged 3–6 years, 575 school-age children aged 7–18 years, 2150 adults aged 19–64 years and 2151 older adults aged 65+years. All finished baseline assessment and started follow-up. Surveillance of ARI symptoms, collection of specimens and laboratory testing are ongoing.
Future plans Findings from this study will be used to provide valuable scientific data to inform ongoing control efforts and future pandemic preparedness for respiratory diseases in China. Planned analyses include analysis of annual pathogen-specific incidence by age group and exploration of healthcare seeking behaviour and factors associated with ARIs and severe ARIs. We will also assess transmission dynamics of common respiratory pathogens in a household transmission subcohort.
- China
- Respiratory infections
- Epidemiology
- EPIDEMIOLOGIC STUDIES
- INFECTIOUS DISEASES
Data availability statement
Data are available upon reasonable request. Deidentified individual participant-level data will be available on reasonable request, upon written request to the corresponding author following publication.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Community Burden of Acute Respiratory Infections in Shanghai (CAReIS) is a 3-year, prospective, age-stratified, community-based longitudinal cohort study to assess the burden of influenza virus, respiratory syncytial virus and SARS-CoV-2 associated community infections in a Chinese population aged 6 months and above.
Our comprehensive laboratory methods (real-time quantitative PCR) help us measure pathogen-specific disease burdens caused by up to 37 respiratory pathogens.
Weekly active follow-up of contacts increases the likelihood of capturing most illness episodes and allows for early collection of samples for microbiological laboratory investigation.
Retention and compliance may be challenging considering the study’s 3-year duration and large number of participants.
A limitation is that CAReIS may not be sufficiently powered to study pathogens less prevalent in the community or explore risk factors associated with severe acute respiratory infections.
Introduction
Acute respiratory infections (ARIs) are a leading cause of morbidity and mortality worldwide.1 2 The great majority of deaths from respiratory infections are caused by lower respiratory tract infections, causing 2.5 million deaths in 2019.3 The COVID-19 pandemic had a profound impact on global health,4 causing 14.83 million excess deaths in 2021.5 With its continuous evolution and adaptation, SARS-CoV-2 has established a biological niche in the human respiratory tract and cocirculates with other endemic respiratory pathogens such as influenza virus, respiratory syncytial virus (RSV) and rhinovirus.6 7 Due to widely used non-pharmaceutical interventions and viral interference,8 changing influenza and RSV epidemiology was observed during the COVID-19 pandemic.7 9–11 Understanding the burden and transmission dynamics of common respiratory pathogens associated with ARIs in a community population can inform ongoing control efforts in communities and guide future interventions (ie, vaccines, diagnostics and therapeutic drugs) in the post-COVID-19 era.
A nationwide sentinel-hospital-based Surveillance for Etiology of Respiratory Infections (SERI) system was established in China in 2009.12 A study prior to the COVID-19 pandemic found that across all age groups, the viruses most frequently causing ARIs were influenza, RSV and rhinoviruses.12 Influenza, RSV and other viral respiratory pathogen activities were significantly suppressed or interrupted during the COVID-19 pandemic.4 9 Most of these SERI-based studies were conducted in hospital settings and focused on the prevalence of aetiological agents in patients, which can be biased by healthcare-seeking behaviour and underestimate disease burden.12 Studies on the incidence of influenza in pregnant women found that community incidences were 0.7, 1.0 and 2.1 per 100 person-months for 2015–2016, 2016–2017 and 2017–2018 seasons, respectively, in Suzhou, China.13 In Jiangsu Province, China, studies conducted during the 2015–2016 and 2016–2017 respiratory virus seasons among individuals aged 60–89 years found that cumulative incidences were 0.8% and 6.1% for influenza-associated ARIs and 0.5% and 1.0% for RSV-associated ARIs.14 These studies focused on special populations (pregnant women and the elderly), investigated only one or two respiratory pathogens and were conducted before the COVID-19 pandemic.13 15 Rigorously conducted prospective cohort studies of multiple respiratory pathogens in large communities are urgently needed to understand true burdens, transmission dynamics and natural histories of influenza, RSV, SARS-CoV-2 and other respiratory pathogens causing ARIs, especially in the post-COVID-19 era.
We designed the Community Burden of Acute Respiratory Infections in Shanghai (CAReIS) study as a 3-year, prospective, age-stratified, community-based longitudinal cohort study to assess the true burden of symptomatic infections caused by influenza virus, RSV and SARS-CoV-2 in a community in Shanghai Pudong New Area District (Pudong, for short). The primary objective was to estimate age-stratified (children aged from 6 months to 18 years, adults aged 19–64 years and elderly aged 65+years) community incidences of influenza virus, RSV and SARS-CoV-2 associated ARIs. Secondary objectives include: (1) investigating community prevalences of influenza virus, RSV and SARS-CoV-2 causing ARI; (2) determining illness course and clinical features by pathogen and age group; and (3) measuring proportions of community ARI cases seeking ambulatory care (outpatient visits and emergency department visits) and proportions of community ARI cases hospitalised (admitted or staying in the hospital for 24 hours or more) by pathogen and age group. Exploratory study objectives include: (1) studying the prevalence of other common respiratory pathogens causing ARI, other than influenza virus, RSV and SARS-CoV-2, in the community; (2) evaluating the proportion of community ARI cases with severe outcomes and factors associated with severe outcomes. We defined severe ARIs as admission to an intensive care unit with any of the following: requiring mechanical ventilation, respiratory failure, acute respiratory distress syndrome, shock or death. We present the study protocol and description of the cohort.
Cohort description
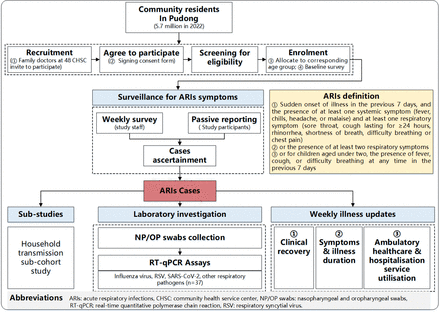
CAReIS is an ongoing prospective longitudinal cohort study being conducted in Pudong, Shanghai, China. The cohort was established in October 2024 and will be followed through September 2027. At enrolment, study staff evaluated the eligibility of potential participants and conducted baseline assessments of sociodemographics, underlying medical conditions, household information, vaccination history and self-rated health data of eligible, consenting individuals. During the follow-up period, events of interest in each participant, that is, symptoms of ARI, are being closely monitored and ascertained, and respiratory samples are obtained from identified ARI cases for timely laboratory testing. Multiplex respiratory pathogen real-time quantitative PCR (RT-qPCR) assays are used to confirm the presence of influenza virus type-A, type-B, RSV, SARS-CoV-2 and other respiratory pathogens in upper respiratory tract specimens from ARI cases. All ARI cases are followed for 28 days after ascertainment to identify illness course and clinical outcome. The primary outcome of the study is the incidence of influenza virus, RSV and SARS-CoV-2 associated ARIs. Secondary outcomes are incidences of medically attended ARIs, including outpatient and emergency department visits and hospital admission caused by influenza virus, RSV or SARS-CoV-2. Exploratory outcomes include incidences of severe ARIs caused by influenza virus, RSV or SARS-CoV-2 and ARIs caused by other common respiratory pathogens investigated in the study. Study activities and procedures are shown in figure 1.
Flow diagram of major study activities.
Study site and population
Our study site, Pudong, is located in subtropical southeast China and is the largest and most populous district in Shanghai City. It had a census-based population of 5.7 million in 2024, among which 1.2 million residents were adults aged 60 years and over. Pudong is a well-developed area served by 47 community health service centres, all of which participate in the study. Influenza vaccine coverage is moderate in Pudong (17.68% in the 2021–2022 season).16 RSV vaccination is not available in the region or China as a whole. Pudong was selected as the study site for several reasons: the local health authority is actively involved, willing to provide support and willing to be responsible for coordinating study activities; vaccination histories can be obtained by linking subjects’ ID to the Immunization Management Information System and to electronic medical records in hospitals via the Regional Healthcare Big Data Center; and Pudong Center for Disease Control and Prevention (CDC) has been involved in SERI studies since 2009 and has experienced, well-trained staff and laboratory technicians to conduct study activities.
The study population includes all permanent residents aged 6 months and above in Pudong, with no gender restrictions. To increase coverage and representativeness, participants were enrolled at all 47 community health service centres by service-centre family doctors. People who were interested in participating in the study came to their family doctor and joined freely on site. Households with multiple members living together were prioritised for enrolment.
Sample size considerations
Our target sample size was 5250 participants, including 250 children aged from 6 months to 2 years, 250 preschool children aged 3–6 years, 450 school-age children aged 7–18 years, 2150 adults aged 19–64 years and 2150 older adults aged 65+years. Target sample sizes were calculated assuming an annual cumulative incidence of symptomatic infection of 30% in infants and preschool children, 20% in school-age children and 5% in adults for influenza virus,17–19 RSV20 or SARS-CoV-2.21 Accounting for loss to follow-up of up to 10%, the sample will ensure achieving a 20% relative precision for incidence estimates at 95% significance levels in each age strata.
Enrolment and baseline assessment
At study initiation, community residents were invited to come to family doctors’ offices for enrolment screening and were enrolled if they met the following eligibility criteria: they (1) were able to understand the study procedures and provide informed consent; (2) could use the internet and mobile devices to complete data collection (for young children and elderly who are illiterate, a surrogate survey is done by their parents/guardians); (3) agreed to have all family household members included in another study (the household transmission subcohort study) if they become confirmed with influenza virus, RSV or SARS-CoV-2 associated ARIs during the study follow-up period; and (4) were permanent residents of the community, defined as persons who resided in the Pudong New Area District for at least 6 months.
Participants with any of the following were excluded: (1) being unwilling or not agreeing to have their medical records or vaccination history accessed by study staff through electronic databases linkage; (2) lacking the ability to adhere to study procedures as prespecified in the study protocol; or (3) planning to leave Pudong for more than 1 month in the following year, regardless of reason (ie, having high risks of losing to follow-up early).
After signing informed consent, study participants were assigned to the appropriate age group and completed baseline questionnaires (online supplemental table 1). Full consent was obtained from all children and adult participants. Older illiterate people gave oral consent, children aged from 6 months to 8 years of age gave written consent from their parents/guardians, while children aged 9–18 years of age gave written consent themselves along with their parents/guardians.
Supplemental material
Surveillance for ARI symptoms
Definition of ARIs
Since enrolment, study participants are being actively followed for 3 years to identify occurrences of ARI. We define an ARI episode as the onset of illness in the previous 7 days and presence of at least one systemic symptom (feeling feverish or having a measured axillary temperature of ≥37.0°C, chills, headache or general malaise) and at least one respiratory symptom (sore throat, cough lasting for ≥24 hours, rhinorrhoea, shortness of breath, difficulty breathing or chest pain); or the presence of at least two respiratory symptoms. For children under 2 years of age or individuals unable to speak, ARIs are defined as the presence of an axillary temperature of ≥37.0°C, cough or difficulty breathing at any time in the previous 7 days or observed during our weekly visits.
We anticipate that participants will experience multiple ARI episodes during the 3 years of the study. To be defined as a new ARI, the onset of new symptoms should be at least 7 days removed from the clinical recovery date of a previous ARI. Clinical recovery is defined as body temperature returning to normal (a measured axillary temperature of <37.0°C) for two consecutive days and complete disappearance of the following symptoms: general malaise, fatigue, cough, nasal congestion or runny nose, sore throat, shortness of breath and difficulty breathing.
Weekly survey and passive reporting
Participants are monitored for ARIs with active surveillance and passive reporting. Active surveillance refers to a weekly survey of participants by study staff, in which the question, ‘since the last contact, have you experienced a common cold or any of the following symptoms: fever, cough, runny nose, sore throat, stuffy nose or body ache?’, is asked. Passive reporting refers to participants voluntarily reporting respiratory symptoms to study staff whenever they experience one.
Case ascertainment
The study staff members verify information reported by participants via phone (online supplemental table 2). For participants who meet our ARI case definition, study staff members encourage the participant to go to their community health service centre within 24 hours to collect respiratory swab samples. If it is inconvenient for the participant to go to the community health centre, a community worker provides door-to-door sample collection service within 24 hours of appointment.
Weekly illness updates and recovery from illness
Identified ARI cases are contacted weekly for 28 days to determine the illness course (ie, symptoms and duration) and clinical recovery (online supplemental table 3), starting from the case ascertainment day. Since by definition ARI cases have symptoms, the weekly survey for ARI symptoms is interrupted until clinical recovery. Instead, during weekly contacts, information on ambulatory healthcare (ie, outpatient/emergency department visits) and hospital service utilisation is obtained (online supplemental table 3). For hospitalised ARI cases, we collect additional information on clinical diagnosis, complications, treatment and drugs used within 1 week after the case has been discharged from the hospital by linking participant ID number to the Pudong New Area Healthcare Big Data System (online supplemental table 4).
Semiannual survey
We will conduct semiannual surveys to update participants’ information that could change during the study, ie, vaccination status, household information and overall health status (online supplemental table 5).
Withdrawals and loss to follow-up
Withdrawals are defined as participants who formally notify study staff that they no longer wish to continue in the study. Participants who do not respond to the weekly symptom survey for two consecutive weeks are contacted via text messages and phone calls, and those who have not responded to study retention outreach for two additional consecutive weeks are de-enrolled. Participants may be re-enrolled if a response is received later. Loss to follow-up of up to 10% was accounted for in the target sample size determination.
Laboratory investigation
Specimen collection
Trained nurses or study staff use sterilised Dacron or nylon swabs to collect upper respiratory tract specimens from participants with ARI symptoms within 24 hours of onset. Two types of specimens are collected: nasopharyngeal swabs and oropharyngeal swabs (NP/OP). Swabs are placed in viral transportation medium tubes (Yocon Biology Technology, Beijing, Batch number: 01240229) and transported within 24 hours of collection to the central laboratory at Pudong CDC, using a cold box to maintain a temperature of 4~8°C.
Processing and storage of specimens
On arrival at Pudong CDC laboratory, swab samples are processed into three aliquots of supernatant. One aliquot is analysed and the other two are retained as backup specimens. Backup specimens are stored at −70°C, and specimens for immediate analysis are stored at 3–8°C until tested.
Laboratory testing
All specimens are subjected to multiplex respiratory pathogen RT-qPCR testing at the Pudong CDC laboratory that can detect 37 respiratory pathogens (online supplemental table 6). Multiplex testing is conducted using microfluidic chip technology combined with RT-qPCR to determine each sample’s relative cycle threshold (Crt) values. Testing is conducted using procedures recommended by the manufacturers, that is, nucleic acid extraction kit (Roche (China) Holding, catalog number: 6369750) and pathogen detection kit (Thermo Fish Scientific, catalog number: 4398986). A Crt threshold of 35 is used to interpret results, with Crt values ≤35 considered positive for a particular pathogen and values >35 considered negative. Test results are reported to study participants within three working days, as indicated in the informed consent document.
Data management and analysis
Data collection
Data and specimens collected at enrolment and baseline and during active follow-up and post follow-up are summarised in table 1. Data collection and questionnaire surveys are performed with an electronic data capture system, the ARIs Information Management System (ARIs-IMS). The software was custom developed for this study. Web-based surveys can be performed on a personal computer desktop or smartphone app. ARIs-IMS supports several key functions, including participant management (recruitment, grouping, follow-up and sample collection appointments), data collection (baseline surveys, ARI symptom reporting and case follow-up), sample collection and testing management (tracking collection, transporting and testing of specimens), test results feedback, data management and storage and role management (authorising access to data and function module).
Data and samples collected in the Community Burden of Acute Respiratory Infections in Shanghai (CAReIS) study, 2024–2027
Immunisation information systems
In addition to self-reported vaccination histories obtained during the baseline and semiannual surveys, participants’ up-to-date vaccination information is obtained by linking the participant’s ID number to the National Immunization Information System. Informed consent for this linkage was obtained from participants at enrolment.
Data linkage and use of electronic medical records
For participants diagnosed with an ARI and hospitalised, their medical records are retrieved within 1 week after discharge by linking the participant’s ID number to the Pudong New Area Healthcare Big Data System. Retrieved data include clinical laboratory results, imaging findings, medications (eg, antibiotics, antiviral treatments), complications and discharge diagnosis. Participants provide informed consent before data are accessed or used at enrolment.
Findings to date
Between 14 October and 22 November 2024, we enrolled 5387 participants into the cohort (table 2). Among the participants, 2595 (48.2%) were male participants and 2792 (51.8%) were female participants. There were 1086 (20.2%) children aged from 6 months to 18 years, 2150 (39.9%) adults aged 19–64 years and 2151 (39.9%) elderly aged ≥60 years. Among the 5387 enrollees, 1038 (19.3%) reported receipt of influenza vaccine in the year before enrolment; 3543 (65.8%) reported a history of COVID-19 vaccination; 611 (11.9%) participants aged 2 years and older reported a history of 23-valent pneumococcal polysaccharide vaccination. Among children aged from 6 months to 5 years, 333 (65.2%) reported receipt of 13-valent pneumococcal conjugate vaccine (PCV13) and 363 (71.0%) reported receipt of Haemophilus influenzae type b conjugate vaccine. All recruited subjects completed their baseline assessments and questionnaires, and we started following participants in November 2024; they are being contacted weekly to monitor ARIs for 3 years.
Characteristics of study participants at baseline
Future plans
Findings from this study will be used to provide up-to-date scientific data on the community burden of specific respiratory infections to inform ongoing control efforts and future pandemic preparedness for respiratory diseases in the post-COVID-19 era in China. We have completed participant enrolment and baseline assessment. Active follow-up, NP/OP swab collection and laboratory testing are ongoing. Planned analyses include: (1) analysis of annual pathogen-specific incidence by age group; (2) characterising clinical presentations, illness courses, clinical recovery and outcome and healthcare services utilisation behaviour of ARI cases; and (3) exploring factors associated with ARIs and severe ARIs, including vaccination history, prior infections and underlying medical conditions. Pathogen-specific incidence rates will be calculated as the number of episodes of influenza virus, RSV or SARS-CoV-2 associated ARIs divided by the total person-time at risk contributed by all study participants during the follow-up period. To make precise rate calculations, the number of days with ARI illness or lost to follow-up will be subtracted from total person-time. The 95% CI of rates will be calculated assuming a Poisson distribution. We will present pathogen-specific incidences by age group (ie, children aged from 6 months to 18 years, adults aged 19–64 years and elderly aged 65+years), vaccination status (influenza vaccines, COVID-19 vaccines and pneumococcal vaccines), season and history of prior infection. Since a wide range of case definitions of ARIs exist in the literature, which can impact on our incidence estimates, we will use the WHO’s ARIs definition22 and recalculate incidences to make comparisons. Factors associated with ARIs and severe ARIs (ie, age, sex, underlying medical conditions, smoking status, having a child in the household, vaccination history and prior infections) will be assessed using a generalised linear mixed model (GLMM) with logit-link function. The GLMM includes a random intercept for each individual nested within each household and community. Seasonality will be controlled by including sinusoidal functions with annual and semiannual cycles for the weeks of the year as fixed effects.
We are also planning to assess the transmission dynamics of common respiratory pathogens in a household transmission subcohort, nested within the primary cohort. During our daily active follow-up, participants with laboratory-confirmed influenza, RSV and SARS-CoV-2 infections and all of their family members will be prospectively enrolled into a subcohort at the time of the index cases’ illness ascertainment. More frequent respiratory specimen (NP/OP swab) sampling and symptom monitoring will be conducted on the household transmission subcohort for up to 28 days, regardless of whether they are symptomatic or asymptomatic. We plan to recruit at least 200 households for each of the three respiratory pathogens (influenza virus, RSV and SARS-CoV-2) in the 3-year study period, in order to assess epidemiological transmission parameters, like incubation period, latent period, generation time, serial interval, infectious period duration, secondary attack rate and proportion of asymptomatic infections, for these respiratory pathogens. Details of the study design of the household transmission subcohorts will be elaborated elsewhere.
Strengths and limitations
The study design has several strengths. First, CAReIS uses a standardised and unified protocol and laboratory procedures, allowing us to generate high-quality community-level incidence data in age strata on a wide range of common respiratory pathogens in the post-COVID-19 pandemic era. The study design ensures accurate data collection and provides insights into the true burden, transmission dynamics, natural history and risk factors of common respiratory pathogen infections. Second, our participants include permanent residents recruited from all 47 community health service centres of Pudong. The sample is representative of the local population and can reflect actual illness occurrence at the community level. Weekly follow-up of contacts increases the likelihood of capturing all illness episodes in the study participants, providing us with precise incidence estimates. Third, the large cohort size (>5200 participants) will allow us to estimate incidence by age group and respiratory pathogen with good statistical precision. The 3-year follow-up period will provide information on year-to-year and season-to-season variation in incidence for most respiratory pathogens, especially influenza virus, RSV and SARS-CoV-2, which have shown a strong seasonal and yearly cyclical pattern in other studies. Fourth, the study uses molecular laboratory methods (ie, RT-qPCR) to investigate up to 37 respiratory pathogens. The laboratory methods used in the study can ensure that the pathogen-specific burden of respiratory infections is measured, which is valuable for developing and optimising targeted interventions (eg, vaccinations) in the future. NP/OP specimens collected from study participants with ARIs can be analysed, sequenced and shared with other investigators for future research purposes. Fifth, our study uses the documented vaccination registry data and electronic medical records maintained by the local health authority. This will allow us to access historical exposure data at different times and various clinical outcomes during hospitalisation. Finally, to facilitate efficient data collection and management, we customised a data information system, ARIs-IMS. Compliance of study personnel and study participants will be significantly increased because of a decreased data collection burden and simplified data collection procedures with ARIs-IMS. Participants are recruited through their family doctors who generally have a strong connection and relationship with the community that will support participant retention in the cohort through weekly contact and semiannual surveys. Family doctors’ efforts will also contribute to high compliance and cohort retention during the 3 years of study follow-up.
This study has limitations. First, we may not identify all respiratory infections through active surveillance of ARI symptoms since our case definitions do not capture atypical and asymptomatic infections. However, we have planned another subcohort (the household transmission subcohort study) nested within our primary cohort. Using this subcohort, we can determine the infection incidence rate and secondary attack rate and, most importantly, the proportion of participants with typical and asymptomatic infections.23 Second, Pudong is a highly developed and densely populated region in eastern China, with high average household income and vaccination coverage levels. Results from this study may not be generalisable to less populated or developed regions in China. Third, our study participants were not randomly selected from the community. They were invited to participate and offered to join freely. Those who have high awareness of his/her health might be more likely to be included in our study, which may bias our estimates as they might tend to report more ARIs than a reference population. Fourth, our study sample size may neither be sufficiently powered to determine the burden of hospitalisation or severe illnesses and explore risk factors of severe outcomes of influenza, RSV and SARS-CoV-2 associated infections, nor can we determine the incidence of some less prevalent pathogens in the community, for example, measles virus and bocavirus. Fifth, specimen test results, whether positive or negative, will be automatically communicated to participants through ARIs-IMS via a smartphone miniprogramme. Study participants can access their information by themselves. Since the study is observational and healthcare seeking behaviour will be decided by the participants themselves, we cannot know how this action will impact the healthcare seeking behaviour of participants. Finally, our laboratory methods and the type of sample specimens collected may have problems for some bacterial agents (eg, Streptococcus pneumoniae, H.influenzae) that commonly colonised the upper respiratory tract. Detection of these pathogens does not necessarily imply infection.
Collaboration
This study is a collaboration between the School of Population Medicine and Public Health, Peking Union Medical College and the Pudong New Area CDC. We are open to collaboration with other researchers to use data generated in this study and to use the platform to conduct further research on influenza virus, RSV and SARS-CoV-2 to develop, evaluate and optimise interventions (ie, vaccinations and treatments). Requests for collaboration should be sent to ZLi (lizhongjiecdc@163.com), accompanied by a detailed protocol and statistical analysis plan. After reviewing for scientific validity, we will contact requestors whose proposals meet the research criteria and for whom an exception does not apply, within 1 month of request.
Data availability statement
Data are available upon reasonable request. Deidentified individual participant-level data will be available on reasonable request, upon written request to the corresponding author following publication.
Ethics statements
Patient consent for publication
Ethics approval
This study was approved by Chinese Academy of Medical Sciences and Peking Union Medical College’s Institutional Review Board (no. CAMS&PUMC-IEC-2024-068).
Acknowledgments
We acknowledge the support of all the subjects who participate in this study, their parents and guardians, the investigators and co-investigators, nurses, physicians, our research staff and other members of the community who are helping us conduct this study.
Footnotes
CY, JY and BZ contributed equally.
Contributors ZLi is the principal investigator on this study who conceived and critically revised the manuscript and is the guarantor of the study. CYe, ZLi, LHao and LRen conceptualised and designed the study. CYe, JYu and BZhao contributed equally to this work. JYu, XYu, YLuo and LXin wrote the first draft. LZhang, YXie, YJia, XZhou, LZhao and YWang contributed to the literature search. BZhao, XWang, YShen and LRen designed laboratory methods. XYu, YLuo and LXin wrote the statistical analysis plan. ZLi, WYang and CYe received funding. YLi, HXin, TZhang, LRodewald, BCowling and WYang commented on and revised drafts of the manuscript. All authors contributed to reviewing, revising and approving the final manuscript and had final responsibility for the decision to submit for publication.
Funding This study is supported by the National Key Research and Development Program of China (2023YFC2308701), the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (2021-I2M-1-044), the CAMS Innovation Fund for Medical Sciences (2023-I2M-3-011), the CAMS Innovation Fund for Medical Sciences (ClFMS) (Grant No. 2023-I2M-2-001), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (Grant No. 2022-ZHCH330-01), the key discipline (GWVI-11.1-02 Infectious Diseases) of the three-year action plan for strengthening the construction of the public health system in Shanghai (2023-2025) and the Pudong New Area Science and Technology Development Innovation fund (No. PKJ2023-Y73).
Competing interests ZLi reports receiving research funding from Ministry of Science and Technology of People's Republic of China. WYang received research funding from Chinese Academy of Medical Sciences (CAMS). CYe reports receiving research funding from Science and Technology Economic Commission and Health Commission of Pudong New Area District, Shanghai. All other authors declare no competing interests.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.