Article Text
Abstract
Objective To examine the feasibility, safety and perceived patient response of a combined repetitive transcranial magnetic stimulation (rTMS) and quadriceps strengthening exercise intervention for knee osteoarthritis.
Methods A two-arm, participant-blinded, therapist-blinded and assessor-blinded, randomised controlled trial with additional follow-up of pain and function at 3 months. Participants were randomised to receive active rTMS+exercise (AR+EX) or sham rTMS+exercise (SR+EX) twice weekly for 6 weeks while completing home exercises twice a week. Primary outcomes included recruitment rate, treatment attendance, dropouts, willingness to undergo therapy (11-point Numeric Rating Scale, ‘not at all willing’=0 and ‘very willing’=10), success of participant, therapist and outcome assessor blinding, adverse events and Global Perceived Effect Scale. Secondary outcomes were pain, function and measures of physiological mechanisms.
Results 86 people were screened, 31 (36%) were randomised, 28 (90%) completed the treatments and 3 (10%) dropouts at 3-month follow-up. Both groups had high treatment attendance (98.4% and 100%). All participants scored at least 7 on the willingness to undergo therapy scale. Blinding was successful. No adverse events were reported. At the postintervention assessment, 80% in the AR+EX group and 75% in the SR+EX group reported an improvement on the Global Perceived Effect Scale. Both groups demonstrated within-group improvements in pain at the postintervention assessment but not at the 3-month follow-up. Function improved only in the AR+EX group at the postintervention assessment.
Conclusions Combined rTMS and quadriceps strengthening exercise intervention for knee osteoarthritis is feasible, safe and well-received. A full-scale trial is justified to assess the clinical benefits of this novel treatment.
Trial registration number ACTRN12621001712897.
- Exercise
- Pain management
- Chronic Pain
- Clinical Trial
- Feasibility Studies
Data availability statement
Data are available on reasonable request. Individual participant data that underlie the results reported in this study—including text, tables, figures and appendices—will be made available following deidentification. This includes deidentified participant data and associated data dictionaries. A published study protocol including planned statistical analysis is available to provide full methodological transparency, along with related supplementary documents. The data will be made available immediately on publication of the study findings, with no end date for access. Access to the data will be granted to researchers for the purpose of conducting secondary analyses. Requests must include the evidence of ethical approval from a recognised Human Research Ethics Committee.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Randomised, assessor-blind, therapist-blind and participant-blind, sham-controlled study design.
Data on the feasibility, safety, analgesic effect and central mechanisms of the combined repetitive transcranial magnetic stimulation and exercise therapy in knee osteoarthritis.
This pilot study was not powered to determine treatment efficacy.
Introduction
Knee osteoarthritis is a leading cause of global disease burden.1 The main symptoms are pain and physical dysfunction that become persistent and debilitating as the disorder progresses.2 Non-surgical, non-drug interventions have been recommended to reduce pain and improve function for knee osteoarthritis.3 Strengthening exercise is the cornerstone of conservative treatment and is recommended as a first-line treatment in all international guidelines.4 5 Exercise yields analgesic effects via both peripheral (ie, improving muscle strength/coordination and joint proprioceptive control that subsequently reduces nociceptive inputs from the affected knee) and central (ie, activating endogenous opioid and pain control systems) mechanisms.6 7 However, the effects of exercise are at best moderate for pain and function, and small for quality of life.8 While knee osteoarthritis is a well-defined joint disorder, pain severity does not always correlate with radiographic findings.9 This discordance has been attributed to maladaptive neuroplasticity of central pain processing pathways.10 Novel treatments targeting the neurophysiological mechanisms underpinning osteoarthritic knee pain could bolster the effects of strengthening exercise and optimise outcomes.
Repetitive transcranial magnetic stimulation (rTMS), a non-invasive brain stimulation technique, might boost the benefits of exercise for knee osteoarthritis. rTMS can induce neuroplasticity, either decreasing (inhibitory, low-frequency stimulation ≤1 Hz) or increasing (excitatory, high-frequency stimulation ≥5 Hz) cortical excitability.11 Research suggests that rTMS alleviates pain via the activation of endogenous opioid pathways of brain regions involved in pain processing.12 High-frequency rTMS applied over the primary motor cortex (M1) has demonstrated superiority to low-frequency rTMS in chronic pain populations.13 Further, as increased M1 excitability is associated with motor learning,14 applying excitatory, high-frequency rTMS over M1 might increase the brain’s responsiveness to the afferent inputs generated by subsequent treatments (ie, exercise), a phenomenon known as ‘priming’.15
Therefore, adding high-frequency rTMS over M1 to strengthening exercise could potentially improve outcomes beyond that which can be achieved with rTMS or exercise alone through two mechanisms: (1) simultaneously modulating peripheral (exercise) and central (rTMS and exercise) mechanisms underpinning knee osteoarthritis pain and/or (2) ‘priming’ the brain to increase its responsiveness to the corticomotor benefits of exercise (ie, increased cortical excitability, enhanced voluntary muscle activation, strength gains, improved motor control).16 Although a recent meta-analysis showed that a combined rTMS and exercise intervention yielded a moderate pain reduction (2 trials, n=38, standardised mean difference=−0.76) for chronic pain conditions in general,17 the effect of this intervention specific to knee osteoarthritis remains unknown. A rigorous and adequately powered randomised controlled trial (RCT) is needed to determine the efficacy of this combined intervention of rTMS and strengthening exercise for knee osteoarthritis. Before conducting a full-scale RCT, a pilot study is recommended to inform the feasibility of the processes essential to the success of a large RCT and the safety of the intervention.18
This study aimed to (1) examine the feasibility, safety and patient-perceived effect of a combined high-frequency rTMS and strengthening exercise intervention for knee osteoarthritis; (2) assess physiological mechanisms underlying the intervention and (3) provide data to conduct a sample size calculation for a fully powered trial based on the results of pain and physical function outcomes.
Methods and analysis
Design
This was an assessor-blinded, therapist-blinded and participant-blinded, two-arm parallel group, pilot RCT. The outcome measures were assessed at baseline and on treatment completion (6 weeks postrandomisation). In addition, pain and function were also assessed 3 months postintervention. The study was prospectively registered (ACTRN12621001712897). The study protocol has been published.19 The study is reported using the Consolidated Standards of Reporting Trials statement extension for pilot trials (online supplemental table S1).20
Supplemental material
Participants
Participants were recruited from the community in Sydney, Australia. Inclusion criteria were as follows: (1) people aged ≥50 years with knee osteoarthritis based on the American College of Rheumatology Clinical Criteria,21 having at least one of the following: morning stiffness <30 min, crepitus, bony tenderness, bony enlargement, no palpable warmth; (2) knee pain for ≥3 months and on most days in the past month and (3) average pain intensity ≥4 on an 11-point Numeric Rating Scale (NRS) in the past week. Exclusion criteria were as follows: (1) previous knee joint replacement or high tibial osteotomy on the affected side; ((2) knee surgery or joint injection in the past 6 months; (3) planned surgery in the next 9 months; (4) using oral corticosteroids currently or in the past 4 weeks; (5) confirmed diagnosis of systemic arthritis (ie, rheumatoid arthritis); (6) previous knee fracture or malignancy; (7) other conditions affecting lower limb function; (8) participating in any knee strengthening exercise for knee osteoarthritis in the past 6 months; (9) loss of sensation of the affected lower limb; (10) neurological or psychiatric disorders; (11) use of neuroactive drugs (eg, tricyclic antidepressant, Clozapine, Foscarnet); (12) contraindications to TMS (ie, epilepsy, metal implant in the skull) using the TMS safety screening questionnaire22 and (13) resting motor threshold (rMT) >80% measured at the baseline assessment, as this would lead to a high stimulating intensity for the rTMS intervention and potential overheating of the coil. Participants were permitted to continue their usual medications during the trial.
Procedures
Potential participants completed an online screening questionnaire to determine eligibility. Eligible participants attended baseline assessment and were randomly allocated to the active rTMS+exercise (AR+EX) or sham rTMS+exercise (SR+EX) group. The assigned treatment was allocated through REDCap prior to the first treatment session, independently of the researchers involved with physiotherapy treatment and outcome assessment. Participants, treating physiotherapists and outcome assessors were blinded to group allocation. All participants received the same instructions and information about rTMS intervention. Participants received either active or sham rTMS immediately before 30 min of one-to-one supervised strengthening exercise twice weekly for 6 weeks (12 sessions). If bilateral symptoms were present, the most painful knee was assessed and treated. Six physiotherapists (at least 2 years’ experience) delivered exercise therapies. All procedures were performed at Neuroscience Research Australia (NeuRA), Sydney, Australia.
Intervention
Repetitive transcranial magnetic stimulation
The rTMS target is the motor hotspot, or the coil position inducing a maximal motor evoked potential (MEP) amplitude measured on electromyography (EMG) using a bipolar surface electrode (Ag-AgCl, Noraxon dual electrodes) on the first dorsal interosseous muscle ipsilateral to the treated knee using a Magstim Rapid2 (Magstim, UK) and a 70 mm figure-of-eight coil. Motor hotspots for the quadriceps muscles were not used as rTMS target as MEPs cannot be reliably elicited at rest,23 and rTMS targeting motor hotspot for the hand has non-somatotopic analgesic effect.24 At each session, 3000 stimuli (10 Hz, 30 trains of 10 s, 20 s intertrain interval) were delivered at 90% of rMT (the minimum intensity at which 5 out of 10 stimuli delivered to the hotspot, evoked an MEP >50 µV).25 rMT was assessed at the beginning of each session. For sham rTMS, a sham coil that looks identical to a real coil but produces no magnetic pulse and only audible clicks was used to deliver the same stimulation protocol as active rTMS.
Exercise
Participants performed standardised quadriceps strengthening exercises (online supplemental table S2) with demonstrated effectiveness for knee osteoarthritis using ankle cuff weights or resistance bands as appropriate.6 8 Each exercise was performed in 3 sets of 10 repetitions with a 30 s rest between sets. The treating physiotherapists determined the starting level and when to progress the exercise based on participants’ feedback and therapist’s clinical judgement. Exercises were progressed as defined in the protocol.19 Participants performed their supervised exercises at home at the same dosage using resistance bands twice per week. Home exercise diaries with instructions were provided for recording the number of sessions, type and number of exercises performed and adverse reactions and collected at the postintervention assessment.
Outcome measures
Primary outcomes
Feasibility, safety and participant-perceived improvement to treatment were measured as: (1) the proportion of participants recruited from the total number screened; (2) the number of sessions attended by each participant; (3) the number of dropouts in each group; (4) willingness of each participant to undergo therapy at baseline on an 11-point NRS with ‘not at all willing’ at 0 and ‘very willing’ at 10; (5) success of participant/outcome assessor/therapist blinding; (6) the number of adverse events and the details of each event; (7) the Global Perceived Effect Scale, where each participant rated their perceived response to treatments on a 7-point Likert scale ranging from ‘completely recovered’ to ‘vastly worsened’.26 The success of participant blinding was assessed at the completion of the intervention using a yes/no response to the question “Do you believe you received real brain stimulation?”’ and an 11-point NRS of the individual’s confidence in that judgement. Participants were also asked “Why do you believe you received the real/sham brain stimulation?” and “Was it divulged to you whether you were receiving real brain stimulation or not?” The success of outcome assessor and treating physiotherapist blinding was determined using a yes/no response to the question “Did you know which intervention group the participant was assigned to before completion of the follow-up laboratory assessment?” and “If you answer “yes”, how was it divulged to you?”.
Secondary outcomes
Pain and function
Knee pain and function were assessed using: (1) an 11-point NRS (0=‘no pain’, 10=‘worst pain imaginable’) for average pain in the past week27; (2) The Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index (24 items (0–4 scale, 0=’none’, 4=’extreme’), total score=96) (Likert V.3.1) and its pain subscale (5 items, total score=20) and physical function subscale (17 items, total score=68), with higher scores indicating worse pain and function28; (3) modified painDETECT Questionnaire (mPD-Q, 7 items, total score=38) to detect a neuropathic pain component (score ≥12) in people with knee osteoarthritis29; (4) the number of painful sites, measured by participants indicating the number of painful sites outside of the affected knee lasting >24 hours in the past week on a four-sided body map (total score=35) with higher scores indicating more widespread hyperalgesia30 and (5) the Pain Catastrophising Scale (PCS, 13 items, total score=0–52) to assess participants’ thoughts and feelings about pain in the domains of magnification, rumination and helplessness, with higher scores indicating higher severity.31 The minimum clinically important change (MCIC) to be detected in knee osteoarthritis trials is 1 unit for pain32 and 6 units for function.33
Physiological mechanism investigations
Corticomotor excitability was measured using TMS mapping.19 Single-pulse TMS was delivered over M1, evoking MEPs recorded on EMG by bipolar surface electrodes over the rectus femoris (RF), vastus lateralis (VL) and vastus medialis oblique (VMO) muscles while participants were seated. EMG signals were amplified (x2000), filtered (20–1000 Hz) and sampled at 2k Hz. Active motor threshold (aMT) was determined on the hotspot for the RF while participants maintained a muscle contraction of 10% averaged root mean square (RMS) EMG of three, 3 s maximal muscle contractions of the RF. During TMS mapping, 126 single-pulse biphasic stimuli (120% of RF aMT, 18 trains of seven stimuli, 2 s interstimulus interval) were delivered pseudorandomly over a 6×7 cm (7 rows and 8 columns) grid using Magstim Rapid2 (Magstim, UK) and a 70 mm figure-of-eight coil, while participants activated the RF to 10% of the averaged RMS EMG of three, 3 s maximal muscle contractions with feedback provided on a monitor. The coil was placed tangentially to the skull with the handle pointing laterally 90°.23 The Neural Navigator (Neurosoft, Russia) was used to track the positions of the TMS coil and participant’s head and ensure stimuli were evenly distributed throughout the grid.
Maps for the RF, VL and VMO muscles were produced offline using a custom script in MATLAB 2023b (MathWorks, USA) based on previously published methods.23 RMS EMG amplitude of MEPs was extracted from a 26 to 46ms window after stimulation, and background RMS EMG (55 to 5ms prior to stimulation) was subtracted. Surface maps within a transformed plane encompassing stimulation coordinates and their corresponding MEP amplitude were generated. The map was then divided into 2744 partitions (49×56), with each partition assigned an estimated MEP amplitude based on the nearest acquired MEP values using triangular linear interpolation. Map volume, a sum of the MEP amplitudes (µV) of all partitions with MEP amplitudes >10% of the maximum MEP amplitude, was used to index corticomotor excitability.
Maximum voluntary isometric contraction (MVIC) of the quadriceps muscles was measured when participants were seated with the hips and knees in 90° flexion using a force transducer. Verbal encouragement was provided. Three attempts were recorded for each participant, and the highest value was used for analysis.
Pressure pain thresholds (PPTs) were assessed using a hand-held pressure algometer (Somedc, Hörby, Sweden, probe size 1 cm2) to quantify mechanical sensitivity. The probe (size 1 cm2) was applied perpendicular to the skin (rate 40 kPa/s) until the participant first reported that the sensation of pressure had changed to pain. PPTs were measured at the side of the knee joint line of the most painful knee and ipsilateral thumbnail. Three measurements at each site were averaged for analysis. PPT assessment has good relative reliability (ICC (intraclss correlation coefficient) =0.83, 95% CI 0.72 to 0.90).34
Conditioned pain modulation (CPM) is a measure thought to reflect endogenous pain inhibition. The CPM response is quantified as a change in the threshold for a stimulus to become painful (test stimulus, TS) at one body site in the presence of pain during a second noxious stimulus (conditioning stimulus, CS) at another body site. In a normal CPM response, painful stimuli at one body site reduce perceived pain intensity induced by noxious stimuli at another body site. PPTs at the upper trapezius muscle contralateral to the painful knee were used as the TS, and the cold pressor test (CPT) in the ipsilateral hand was used as the CS. Three PPTs (TS1) were measured before the CPT. For CPT, participants immersed the hand in cold water (4°C) for a maximum of 2 min.35 Three PPTs (TS2) were reassessed when CPT-evoked pain reached 50 on a NRS (0–100). If the pain became unbearable, participants were permitted to remove their hand before completing the CPT, and a pain rating was obtained immediately after participants removed their hand. The magnitude of CPM was determined as (1) absolute value: TS2 minus TS1; and (2) per cent change: [(TS2−TS1)/TS1]×100, where a positive value indicated normal descending pain inhibitory function.36 CPM paradigm has shown good relative reliability (ICC>0.75).37
Statistical analysis
Although a sample size calculation is not required in a pilot RCT, 15–20 participants per treatment arm is recommended.19 We selected a sample size of a total of 30 participants as we successfully completed a previous pilot RCT with a similar design.16 As a pilot study has low power, between-group statistical comparisons were not conducted.38 Participant demographics and primary outcome measures were analysed and reported descriptively (mean and SD or percentages). A full-scale RCT would be deemed to be feasible if the following predefined criteria thresholds are met: (1) attendance rate >80%; (2) dropout rate <20%; (3) 80% of participants scored ≥7 on the 11-point willingness to undergo therapy scale at baseline.19 For secondary outcome measures, within-group changes were calculated as follow-up minus baseline assessments (mean and 95% CI). Between-group differences (mean and 95% CI) were also calculated at postintervention and 3 months. Two-sided t-tests were used for within-group comparisons between baseline and follow-up measures, and effect sizes (Cohen’s d, 0.2 as small, 0.5 moderate and 0.8 large) were calculated. All analyses were conducted using R, V.4.03 (R Development Core Team, Vienna, Austria).39
Results
Feasibility
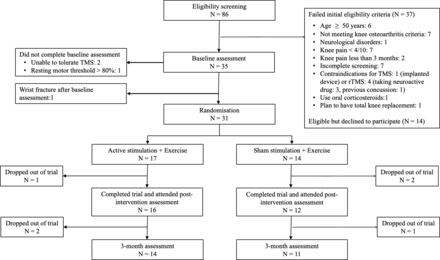
Between June 2022 and August 2023, 86 people were screened for eligibility, 35 (41%) were eligible and attended baseline assessment. Three participants were excluded at baseline assessment, and one withdrew after baseline assessment due to a wrist fracture unrelated to the study (figure 1). 31 participants (36% of screened participants) were enrolled and entered randomisation (AR+EX group N=17; SR+EX group N=14). All participants (100%) scored ≥7 on the willingness to undergo therapy (table 1). The dropout rate was 10% at postintervention assessment. In the AR+EX group, one participant withdrew due to work commitments. In the SR+EX group, one participant withdrew due to a flare-up of knee pain after the first treatment and another due to travelling distance. The dropout rate was 19% at 3 months (AR+EX group: N=3; SR+EX group: N=3). The treatment attendance rate was 98.4% (11.8±0.54 sessions) in the AR+EX group and 100% in the SR+EX group. No participant reported that treatment allocation was revealed before completing the postintervention assessment. 13 participants (81%) in the AR+EX group and 3 (25%) in the SR+EX group correctly guessed their treatment group. In the AR+EX group, 11 participants thought they received ‘real’ rTMS because their symptoms improved, and for the other two participants, because of perceived ‘stimulation’ sensations in the hand or knee during rTMS. The outcome assessor and physiotherapists reported the treatment group allocation was not divulged before the trial completion.
Baseline characteristics of participants (mean and SD)
Flow of participants through the trial. rTMS, repetitive TMS; TMS, transcranial magnetic stimulation.
Safety
No adverse event related to rTMS was reported. The AR+EX group reported mild side effects during rTMS: two episodes of transient feelings in a tooth filling and two episodes of transient sensation on the face. These side effects did not impact rTMS and exercise treatment completion. One participant in the ST+EX group experienced an acute flare-up of knee pain after the first treatment and subsequently withdrew from the study. This acute episode of knee pain was attributed to strengthening exercise as it is unlikely that sham rTMS would yield negative effects on pain.
Participant-perceived improvement
On treatment completion, 13 (80%) participants in the AR+EX group and 9 (75%) in the SR+EX group reported an improvement in their symptoms (figure 2). One participant in each group reported worsened symptoms after treatment.
Percentage of participants reporting perceived change across categories from ‘vastly worse’ to ‘completely recovered’ after 6-week interventions. rTMS, repetitive TMS; TMS, transcranial magnetic stimulation.
Pain and function
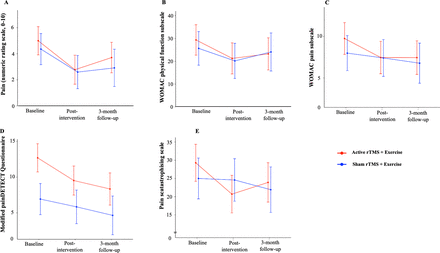
Average pain (11-point NRS) in the past week reduced after the 6-week intervention in both groups (AR+EX group: p<0.01, d=1.34; SR+EX group: p=0.03, d=1.07) but did not change between baseline and 3 months (p>0.11) (figures 3 and 4) (table 2). WOMAC physical function subscale score improved after intervention in the AR+EX group (p=0.02, d=1.02) but not the SR+EX group (p=0.23). WOMAC physical function subscale score did not change between baseline and 3 months in either group (p>0.12).
Group data (mean and 95% CI) for pain and functional outcomes
Pain and function (mean and 95% CI) at baseline, postintervention and 3-month follow-up. ((A) Average pain in the past week; (B) WOMAC physical function subscale; (C) WOMAC pain subscale; (D) modified painDETECT Questionnaire; (E) Pain Catastrophising Scale). rTMS, repetitive transcranial magnetic stimulation; WOMAC, The Western Ontario and McMaster Universities Osteoarthritis Index.
Within-group changes in pain and function preintervention and postintervention. ((A) Average pain in the past week; (B) WOMAC physical function subscale; (C) WOMAC pain subscale; (D) modified painDETECT Questionnaire; (E) Pain Catastrophising Scale). rTMS, repetitive transcranial magnetic stimulation; WOMAC, The Western Ontario and McMaster Universities Osteoarthritis Index.
WOMAC pain subscale score reduced at postintervention (p=0.03, d=0.97) and at 3-month follow-up (p=0.04, d=0.97) in the AR+EX group but did not change in the SR+EX group (p>0.83). mPD-Q score reduced at postintervention (p=0.04, d=0.89) and at 3-month follow-up (p<0.01, d=1.23) in the AR+EX group but did not change in the SR+EX group (p>0.74). The PCS score reduced at postintervention (p<0.01, d=1.54) and at 3-month follow-up (p=0.046, d=0.97) in the AR+EX group but did not change in the SR+EX group (p>0.98). The number of painful sites did not change within groups at any time points (p>0.18).
Physiological mechanisms
Map volume for quadriceps muscles was unchanged after intervention in both groups (p>0.18), except for an increase in the VL muscle in the SR+EX group (0.99 mV, 95% CI −0.05 to 1.93, p=0.047, d=0.90) (online supplemental table S3). MVIC was unchanged after intervention in both groups (p>0.18). PPTs were unchanged in both groups at the knee (p>0.30) and the thumb (p>0.34). Similarly, CPM was unchanged in both groups (p>0.45).
Sample size calculation
A study with 55 participants per arm would achieve 80% power considering a two-sided significance level of 0.05 and a correlation between premeasurements and postmeasurements of 0.21 for pain. Accounting for a 20% dropout rate, a total of 138 participants would be required to detect the minimum clinically important between-group difference of 1.8 units for pain.33
Discussion
This is the first study to evaluate the addition of rTMS to quadriceps strengthening exercise in knee osteoarthritis. The findings suggested the combined intervention is feasible, safe and well received to this population, and adding rTMS to quadriceps strengthening exercises might improve pain and function in knee osteoarthritis. Thus, our results support a definitive trial to examine the effects of this intervention on the symptoms in knee osteoarthritis.
Attendance was nearly 100% for treatments and 90% for the postintervention assessment, and all participants rated ≥7 on the willingness to undergo therapy. These findings met our predetermined criteria thresholds,19 supporting the feasibility of a full-scale clinical trial. Although the dropout rate at the 3-month follow-up was 19%, a full-scale trial with more resources could reduce the dropout rate. The proportion of participants who thought they received active rTMS in both groups (AR+EX 81% vs SR+EX 75%) was similar. A recent study applying electrical stimulation synchronised to rTMS pulses on the head, mimicking scalp tapping sensation induced by active rTMS, for all participants, reported that 58% in the active rTMS and 44% in the sham rTMS groups thought they received active treatments.40 Similar to that study, most of our participants based their judgement on perceived analgesic effects. Future trials might consider this approach to strengthen participant blinding. Adverse reactions to rTMS during (eg, seizure, syncope) and after (headache or pain at the stimulation site, hearing-related complaints) stimulation were reported previously, although occurring rarely (eg, 0.1% for seizure).41 No participant reported rTMS-related adverse reactions in this study. One participant in the SR+EX group reported an adverse reaction (flare-up of knee pain) attributed to exercise after the first treatment and discontinued the study. Our incidence rate of adverse reactions is lower than previous findings for the rTMS (ie, 15% headaches)13 or exercise therapy (23%–30%).42 Generally, we found no barriers to the implementation of the interventions or outcome measures, and the rTMS and exercise intervention appears to be safe and well tolerated.
Participants received 12 supervised exercise sessions recommended for knee osteoarthritis43 over 6 weeks. Notably, recent meta-analyses found that at least 3 months of strengthening exercise are needed to improve pain and disability in this condition, regardless of exercise volume (ie, frequency, intensity).44 Future definitive trials may consider a 3-month intervention duration. We did not identify any issue with the rTMS protocol. A recent RCT demonstrated that a 22-week rTMS intervention of the same rTMS parameters (15 sessions) had long-term analgesic effects on chronic neuropathic pain.40 The authors suggested the efficacy could be attributed to the cumulative effects of rTMS sessions over time, further supporting a longer intervention duration in future trials.
Our results of pain outcomes suggest that AR+EX might induce larger and longer-lasting analgesic effects than SR+EX. At postintervention assessment, the AR+EX group demonstrated improvements in pain (11-point NRS) and physical function (WOMAC physical functional subscale) exceeding the MCIC for these outcomes, whereas the SR+EX group only improved in pain and this improvement was below the MCIC. Further, WOMAC pain subscale, mPD-Q and PCS scores at the postintervention assessment and at 3-month follow-up suggest that adding rTMS to quadriceps strengthening could lead to long-term benefits for osteoarthritic pain, neuropathic-like pain (measured by the mPD-Q) and pain catastrophisation (measured by the PCS) in knee osteoarthritis. Notably, the baseline mPD-Q score in the AR+EX group was higher than the SR+EX group (see figure 3). Based on the cut-off points for mPD-Q,29 the AR+EX group displayed a possible neuropathic pain profile (13–18) whereas the SR+EX group displayed a nociceptive pain profile (≤12). While a recent clinical trial has demonstrated the efficacy of rTMS in chronic neuropathic pain,24 whether this combined intervention is more efficacious in people with a neuropathic component of osteoarthritic knee pain cannot be inferred in this pilot study. To evaluate clinical efficacy of a combined rTMS and strengthening intervention on pain and physical function for knee osteoarthritis, full-scale trials may consider a sample size of 138, 12 treatment sessions over 3 months and assessing the primary outcomes of pain (11-point NRS) and physical function (WOMAC physical function subscale) at baseline and 3 months postintervention.
rTMS can induce long-lasting neuroplastic changes (ie, decreasing or increasing cortical excitability) by modulating N-methyl-D-aspartate receptor activity, hypothesised as the underlying mechanism of analgesic effects.45 46 Despite improvements in pain and function, the AR+EX group (10 Hz M1-rTMS) did not display an increase in corticomotor excitability observed in previous research.46 Another study also showed a pain reduction but no change in corticomotor excitability after 10-Hz M1-rTMS (five consecutive days).47 It is likely that the analgesic effects of rTMS might be driven by neuroplastic effects at remote cortical regions connecting to M1, not M1 itself, unrelated to modulating corticomotor excitability and that were not measured here.47 Future studies should evaluate rTMS-induced neuroplastic changes using other measures (ie, altered brain oscillations on electroencephalography) and their relationship with pain outcomes.48 Further, increased quadriceps strength, reduced pressure pain sensitivity and improved descending pain inhibition after quadriceps strengthening exercises (alone or with adjunct treatments) were reported in knee osteoarthritis.16 49 However, we found no changes in MVIC, PPTs and CPM in either group, regardless of observed within-group changes in pain and function. It is plausible that a longer intervention duration might be necessary to induce physiological changes similar to previous research. Alternatively, the interventions might act through other mechanisms such as placebo, pain catastrophisation or other pain-related psychological factors. As this is a feasibility study, future full-scale studies are needed to determine underlying physiological mechanisms of this novel intervention in knee osteoarthritis.
Limitations
This study has some limitations. First, this pilot RCT was not powered to determine clinical efficacy, effects of the combined intervention of rTMS and strengthening exercise on pain and function in knee osteoarthritis cannot be inferred. Second, while self-reported WOMAC (physical function subscale) was used to assess function, objective outcome measures of physical function were not included in this study. The 2013 OARSI (Osteoarthritis Research Society International) consensus recommends a set of performance-based tests for physical function in people with knee osteoarthritis.50 According to this consensus, a minimal core set of three tests (ie, 30 s chair-stand test, 40 m fast-paced walk test and stair-climb test) should be included as outcome measures to complement patient-reported measures in future large clinical trials.
In conclusion, data from this pilot study support a definitive trial examining a combined rTMS and quadriceps strengthening exercise intervention for knee osteoarthritis. Despite no identified barriers to implementing this study methodology in future trials, a 3-month intervention duration should be considered to yield long-term benefits. Based on our findings, a fully powered clinical trial is justified to evaluate the clinical benefits of this novel treatment in knee osteoarthritis.
Patient and public involvement
We engaged a consumer representative from the Musculoskeletal Health Clinical Academic Group Consumer Community Council, Australian and New Zealand Musculoskeletal Clinical Trial Network and received feedback on the study including the proposed intervention and potential barriers to participant recruitment. The feedback from the consumer representative was used to guide the design of intervention and recruitment strategies.
Data availability statement
Data are available on reasonable request. Individual participant data that underlie the results reported in this study—including text, tables, figures and appendices—will be made available following deidentification. This includes deidentified participant data and associated data dictionaries. A published study protocol including planned statistical analysis is available to provide full methodological transparency, along with related supplementary documents. The data will be made available immediately on publication of the study findings, with no end date for access. Access to the data will be granted to researchers for the purpose of conducting secondary analyses. Requests must include the evidence of ethical approval from a recognised Human Research Ethics Committee.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by the University of New South Wales Human Research Ethics Committee (HC210954). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We would like to acknowledge the contribution of Carley Robertson, Skye McFadyen, Tammy Wells and Dr Lloyd Chen to this study as the trial physiotherapists.
References
Footnotes
X @iamedwardgorgon, @EdelOH
Contributors W-JC, SA, JMN and SMS were involved in the conception and design of the study protocol. W-JC, SA, JMN, NC, HF, RRNR, EG, EO'H and SMS contributed to methodology of the study. W-JC, AC, NC, EG and RRNR were involved in data collection. W-JC analysed data and drafted the manuscript. All authors edited, reviewed and approved the manuscript. Guarantor: W-JC.
Funding This work is supported by the Australian and New Zealand Musculoskeletal Clinical Trial Network (Seed Granting Award).
Disclaimer The funding body does not have a role in study design and will not have a role in study execution, data analyses and interpretation or decision to submit results.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.