Article Text
Abstract
Objectives This study aims to compare the outcomes of different pharmacotherapies for subjective tinnitus patients using a comprehensive network meta-analysis.
Design Systematic review and network meta-analysis.
Data sources PubMed, EMBASE, Web of Science and CINAHL Complete databases were searched from inception until 6 March 2025.
Eligibility criteria Randomised controlled trials (RCTs) comparing pharmacotherapy treatment effects for subjective tinnitus patients were included with tinnitus severity as the primary outcome, and annoyance and tinnitus loudness as secondary outcomes.
Data extraction and synthesis Two reviewers independently retrieved and screened full articles using a standardised and prepiloted Excel form. Network meta-analysis was conducted with heterogeneity, study risk of bias (ROB), risk of publication bias and certainty of evidence examined using I2, τ2, ROB2, funnel plots and Grading of Recommendations, Assessment, Development, and Evaluation assessments.
Results Sixty full-text RCTs from 21 countries were included in the analysis with 22% low ROB, 58% some concerns and 20% high ROB. The heterogeneity parameter I2 was 0.67 (95% CI 0.33 to 0.84), 0 (95% CI 0 to 0.9) and 0.63 (95% CI 0 to 0.89) for the severity, annoyance and loudness network analysis, respectively. The only significant publication bias assessment by Egger’s test was detected in the loudness network analysis (p<0.05). Ginkgo biloba with vitamin (standardised mean differences (SMD): −3.11, 95% CI (−4.15 to –2.06)), acamprosate (SMD: −0.88, 95% CI (−1.81 to –0.04)) and fluoxetine (SMD: −3.28, 95% CI (−4.23 to –2.34)) ranked first in severity, annoyance and loudness, respectively, compared with placebo. There are three significant inconsistent comparisons observed in the severity network and ranked with very low certainty of evidence by GRADE assessment.
Conclusions This meta-analysis found that antioxidant supplementation, such as Ginkgo biloba, and vitamins and gamma-aminobutyric acid agonists, represented by acamprosate, could be promising treatments for subjective tinnitus. Further trials with rigorous design and larger sample sizes are necessary to supplement the current evidence.
Trial registration number INPLASY202480066.
- Meta-Analysis
- OTOLARYNGOLOGY
- CLINICAL PHARMACOLOGY
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This network meta-analysis synthesised 60 full-text randomised controlled trials from 21 countries with no language restrictions, enhancing global generalisability and minimising selection bias.
Methodological rigour was ensured through adherence to Preferred Reporting Items for Systematic Reviews and Meta-Analyses-Network Meta-Analysis guidelines, prespecified subgroup/sensitivity analyses and comprehensive assessments, aligning with Cochrane recommendations.
Multiple outcomes were evaluated, identifying Ginkgo biloba with vitamin, acamprosate, and fluoxetine as top-ranked interventions compared to placebo.
High heterogeneity and limited reliability due to 78% of studies having some concerns/high risk of bias, alongside significant inconsistencies in severity comparisons.
Exclusion of grey literature, lack of safety outcome evaluations and incomplete assessment of measurement scale sensitivity may constrain clinical applicability, as highlighted by publication bias in loudness outcomes (Egger’s test: p<0.05).
Introduction
Tinnitus, a prevalent audiological condition, is characterised by the perception of a ringing or buzzing sound in the absence of a corresponding auditory source.1 2 It can result from various factors, including ageing, noise, ototoxicity drugs and head and neck trauma.3 While the exact processes driving tinnitus remain incompletely comprehended, abnormal neural activity and connectivity in both auditory and non-auditory pathways might play a vital role.4 5 Tinnitus can be classified as subjective or objective based on the presence of pulsative perception, as acute, or chronic based on the tinnitus duration.6 Subjective non-pulsative tinnitus is thought to impact as much as 21% of the overall adult population, with this number rising to potentially 30% among adults aged 50 and above.7 Subjective tinnitus can significantly impact patients’ quality of life, causing sleep disturbances, concentration difficulties and emotional distress.8
Currently, several pharmacotherapies have been proposed for tinnitus management, including steroid administration,9 drugs that enhance gamma-aminobutyric acid (GABA) function (GABA agonists),10 antidepressants (fluoxetine),11 herbal medicines (Ginkgo biloba, GB)12 among others.13 Chung conducted a meta-analysis concluding that intratympanic dexamethasone injection (ITDI) alone did not exhibit a significant effect in managing tinnitus when compared with placebo and recommended the combination treatment of ITDI with other drugs.14 Additionally, GABA agonist drugs are expected to reduce neuron excitability and alleviate tinnitus symptoms as tinnitus has been associated with a decrease in GABA-mediated inhibition in various brain regions.10 As a significant number of tinnitus patients also experience symptoms of depression, antidepressants are also suggested as a treatment for chronic tinnitus.15 16 Nonetheless, as pointed out by Langguth, a broad range of medications with diverse medical purposes have been used off-label, but the efficacy of these pharmacotherapies remains unassessed comprehensively, highlighting the lack of a universally recognised effective treatment for subjective tinnitus.17 Few guidelines worldwide showed positive recommendations for the pharmaceutical treatment of tinnitus. German guidelines in 2022 concluded that there are no sufficient data on the effectiveness of drug treatment targeting tinnitus specifically.18 19 The earlier guidelines either recommend against or weakly recommend against a pharmaceutical prescription for tinnitus in the USA, Europe and Japan.1 20 21 The current scope of pharmaceutical treatment for tinnitus could benefit from a systematic and extensive review covering efficacy evaluation and evidence assessments.
Given the various treatment options and evidence evaluation challenge, a network meta-analysis (NMA) approach is preferred, which offers a promising avenue for assessing the efficacy of different medical treatments.22 NMA combines direct and indirect evidence from clinical trials, enabling simultaneous comparison and ranking of multiple interventions, thus providing an overview of their relative effectiveness.23 We aim to conduct a comprehensive systematic review and evaluation of pharmacotherapies for subjective tinnitus using randomised controlled trials (RCTs) focusing on alleviating the tinnitus severity, annoyance and loudness.
Materials and methods
Search strategy and study selection
This study adheres to the Preferred Reporting Items for Systematic Reviews and Meta-analyses reporting guideline24 and in accordance with the Cochrane Handbook.25 26 The study was registered in INPLASY (Register No. INPLASY202480066). We conducted a comprehensive search across four electronic databases: PubMed, EMBASE, Web of Science and Cumulative Index to Nursing and Allied Health Literature (CINAHL) Complete from interception to 6 March 2025, without language restriction.
The search approach was based on the ‘PICOS’ tool, which served as the foundation for defining the inclusion criteria. The following criteria were used: (P) Population: adults with tinnitus including idiopathic subjective non-pulsative tinnitus, acute and chronic tinnitus; those focused on tinnitus with noise-induced or trauma-induced sudden hearing loss or deafness, vestibular disorders were excluded; (I) Intervention: pharmaceutical treatments; (C) Comparator: other drug treatments or placebo; (O) Outcomes: the primary outcome is the change in the severity of the tinnitus; the secondary outcomes include the change in annoyance and tinnitus loudness and (S) Study type: RCTs; conference abstracts, open-label studies were excluded for the data completeness and blindness bias. The specific search strategies for each database were included in online supplemental table 1.
Supplemental material
Data extraction
Two authors (PL and CC) independently retrieved and screened full articles based on the selection criteria. Any discrepancies were first attempted to resolve through discussion and consensus in a meeting between the two authors. If consensus could not be reached through discussion, a third senior reviewer (SS) was consulted to provide an independent assessment and help resolve the disagreement. The third reviewer’s decision was considered final and binding to ensure consistency and objectivity in the review process. For the studies written in languages other than English or Chinese, we implemented a comprehensive translation protocol. Non-English studies were translated into English by qualified translators who possessed specialised knowledge in the subject area. The translation accuracy was further ensured by having a second independent translator cross-check and validate the translated content. Any discrepancies were discussed between the two translators and the senior scholar (SS). Data extraction was performed using a standardised and prepiloted Excel form. The following information was recorded under specific headings: author, year of publication, study design and tinnitus details including tinnitus type, duration, patient age, pharmaceutical interventions, sample size, route, follow-up duration, adverse event, outcomes and its measurement method, intention-to-treat or per-protocol (PP) methods used and outcomes.
Risk of bias and evidence quality assessment
Two reviewers (PL and CC) independently evaluated the risk of bias (ROB) in RCTs following the guidelines outlined in the Cochrane Handbook using the ROB2 Excel tool. The assessment considered the following five domains: (1) selection bias, (2) performance bias, (3) detection bias, (4) attrition bias and (5) reporting bias. Each domain was rated on a scale of low ROB, some concern or high ROB. The overall quality of the study regarding the main outcome was determined following the Cochrane handbook25: low ROB if all domains are low ROB; some concerns if at least one domain some concern but no high ROB for any domain; high ROB in at least one high ROB domain.
The quality of evidence for each outcome is assessed following the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) handbook27 and determined independently by two investigators (PL and CC), which consists of the following criteria: (1) ROB: evaluating the methodological quality of studies; (2) Inconsistency: assessing the variability of results across studies; (3) Indirectness: determining if the evidence applies to the population, intervention, comparator or outcome of interest; (4) Imprecision: evaluating the width of CIs and the sample size; (5) Publication Bias: considering whether there is evidence of selective reporting or missing studies. Evidence from RCTs starts as high-quality (represented by ⨁⨁⨁⨁) but can be downgraded based on the above factors (moderate ◯⨁⨁⨁, low ◯◯⨁⨁ or very low ◯◯◯⨁). In case of any disparities during the ROB and GRADE assessment process, the two investigators would first discuss and turn to the senior investigator (SS) if opinions are not aligned.
Statistical analyses
Network meta-analyses were conducted to compare different pharmaceutical treatment strategies by the R software (V.4.1.3). For each outcome, network plots were first generated to visualise the network, with interventions represented as nodes and node size indicating the corresponding patient number. The edges on the plots represent the number of studies. Then, the results were evaluated by calculating the pooled estimates of the risk ratio for dichotomous outcomes or standardised mean differences (SMD) for continuous outcomes with a 95% CI, which makes the different results with different scales and questionnaires comparable. The decreased values of outcomes were recorded for the analysis.
The authors used the frequentist NMA with the R package ‘netmeta’.28 The fixed effect model was first assessed for heterogeneity in the network meta-analysis and adopted when I2 values < 50. When I2 values ≥ 50% as heterogeneity indicated, the random effect model was chosen.29 30 Other statistical parameters of heterogeneity (within treatment contrasts) and inconsistency (between treatment contrasts) such as 2τ and Q values were also documented. Furthermore, we evaluated the detail inconsistency between indirect and direct comparisons using node-splitting analysis.31 A League table was created showing the SMD and CI for all treatment contrasts facilitating direct and indirect pairwise comparisons. Treatments were further ranked using the surface under the curve cumulative ranking probabilities (SUCRA) and the treatment effect was illustrated with forest plots compared with placebo. For sensitivity analyses, we systematically evaluated the robustness of our meta-analysis results by assessing the impact of excluding each study (leave one out) and the PP results. For subgroup analyses, we will examine the effect size for primary outcome across predefined subgroups based on chronic tinnitus, ROB and measurement scales. To assess the publication bias in the meta-analysis, we employed comparison-adjusted funnel plots and Egger’s test.32 The five treatment comparisons with the largest SMDs were labelled. Statistical significance was determined using a p-value threshold of 0.05.
Patient and public involvement
Patients and members of the public were not involved in the design, conduct, reporting or dissemination plans of this meta-analysis.
Results
Literature search
The initial pool of studies consists of 3272 articles obtained from the aforementioned databases and 2087 duplicated articles were removed. Inappropriate articles were also excluded for reasons shown in online supplemental figure 1, including those that are not RCTs and used interventions other than medicine. The remaining 100 unique studies underwent full-text screening. After filtering 40 articles, 60 full-text articles were included in the analysis eventually. The included studies were published from 1993 to 2025 and the patients’ average age is 52 with varying follow-up lengths. They are from 21 countries with Iran (nine studies) published the most followed by the USA (eight studies), Brazil (six studies) and Japan (five studies). Further detailed information is found in online supplemental table 2.
Supplemental material
Supplemental material
ROB assessment
The overview of the ROB for the main outcome in the included RCT studies shows that the majority of RCTs demonstrated some concerns (35, 58%) (online supplemental figure 2). Among the 60 RCTs, 12 (20%) studies were classified as high ROB and 13 (22%) studies were of low ROB. A detailed assessment of ROB for each study is illustrated in online supplemental table 3 regarding each domain.
NMA results for severity
There are 53 studies reporting severity but several studies could not be used for the NMA since the incompatible data, including two papers from Elzayat et al33 34 and one from Kucher et al35 with effective rates data, Koybasi et al36 and Ledesma et al37 with correlation results, Nishad et al38 without SD data. Two studies cannot be connected to the network.11 39 Thus, the data from 45 studies and 3491 patients were included in the analysis. We chose the most effective intervention arm for the Suckfull et al40 since there are three Neramexane arms with different doses. Specifically, most of the studies (31/45) implemented the tinnitus handicap inventory (THI) as measurement scale and among them. The original THI questionnaire brought up by Newman et al41–43 is the most acknowledged one. There are also local-adapted or translated versions existing, including Arabic,44 Turkish,45 Brazilian Portuguese,46 Korean47 and Persian.48
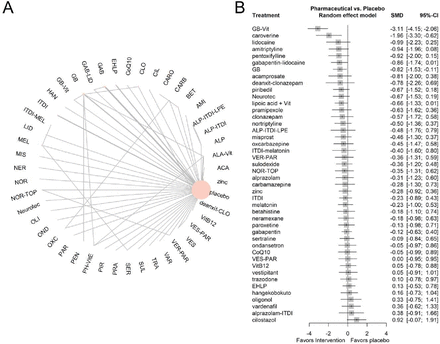
The network plot shows that most of the analysis was connected through the placebo treatment arm, which means the comparison results between treatments other than placebo would be generated mainly by indirect analysis data (figure 1A). As shown in figure 1B, we used a forest plot for treatment efficacy illustration, and the GB and vitamins ranked the most effective treatment with SMD: −3.11, 95% CI −4.15 to –2.06. Caroverine and lidocaine follow as the second and the third with SMD: −1.96, 95% CI −3.29 to –0.63 and SMD: −0.99, 95% CI −2.33, 0.25. The detailed league table showed all direct and indirect comparison results (online supplemental table 4). The SUCRA results echoed the forest plot with the first five treatments as GB and vitamins, caroverine, amitriptyline and lidocaine with SUCRA scores of 0.99, 0.94, 0.77 and 0.77, respectively (online supplemental table 5). The heterogeneity parameter I2 was 0.67 (95% CI 0.33 to 0.84) with τ2 of 0.15 (online supplemental table 6). The within-design heterogeneity and between-design inconsistency presented by the Q value were 6.63 (p=0.16) and 17.51 (p<0.01) (online supplemental table 7). Furthermore, we conducted the node-splitting analysis to evaluate the inconsistency. Only comparisons with both direct and indirect were presented for illustration and analysis as the evidence between treatments without placebo is mainly indirect results (online supplemental figure 3, online supplemental table 8). There are 10 comparisons with both direct and indirect evidence and 3 among them showed significant inconsistency. Notably, these comparisons were derived from four studies with three high ROB43 47 49 and one low ROB50 and down-rated as very low in GRADE assessment (online supplemental table 8). The rest evidence, though with insignificant inconsistency, still negatively affected by the small number of trials and patients and assessed as low quality of evidence (online supplemental table 8). We also assessed the publication bias based on the funnel plot. Publication bias, where positive or statistically significant results are more likely to be published, may skew meta-analytic conclusions, particularly when small studies with exaggerated effects dominate the analysis. The funnel plot showed a generally symmetric shape and an insignificant publication bias as Egger’s test (p=0.43) (online supplemental figure 4).
(A) Network structure of changes in severity of tinnitus. (B) Forest plot of the changes in severity of tinnitus. The effect size <0 indicates the treatment was associated with higher improvement in severity of tinnitus than the placebo. ACA, acamprosate; ALA, α-lipoic acid; ALP, alprazolam; AMI, amitriptyline; BET, betahistine; CARB, carbamazepine; CARO, caroverine; CIL, cilostazol; CLO, clonazepam; CoQ10, coenzyme Q10; EHLP, enzymolyzed honeybee larvae; GAB, gabapentin; GB, ginkgo biloba; HAN, hangekobokuto; ITDI, intratympanic dexamethasone injection; LID, lidocaine; LPE, lipo-prostaglandin E1; MEL, melatonin; MIS, misprost; NER, neramexane; NOR, nortriptyline; OLI, oligonol; OND, ondansetron; OXC: oxcarbazepine; PAR, paroxetine; PEN, pentoxifylline; PH, papaverine hydrochloride; PIR, piribedil; PRA, pramipexole; SER, sertraline; SUL, sulodexide; TOP, topiramate; TRA, trazodone; VAR, vardenafil; VER, verapamil; VES, vestipitant; Vit, vitamin.
NMA results for annoyance
There are 16 studies reporting tinnitus annoyance. Among them, Kucher et al35 only provided adjusted means, which could not be included for further analysis. Moreover, the intervention arms of Meeus et al,51 Prochazkova et al52 and Han et al53 were excluded as they could not be connected. Twelve studies and 12 interventions were included with 1059 patients (figure 2A). As for the measurement questionnaire, five studies54–58 used 100 score scale while six used 10 score scale.40 48 59–62 With the fixed effect model, the acamprosate achieved the most annoyance decrease (SMD: −0.88, 95% CI −1.81 to 0.04) followed by Neurotec (SMD: −0.69, 95% CI −1.09 to –0.29) and sertraline (SMD: −0.58, 95% CI −0.92 to –0.24) (figure 2B). The league table provided all direct and indirect comparison results (online supplemental table 9). The SUCRA scores mirrored the forest plot, with the top three treatments being acamprosate (0.88), Neurotec (0.86), sertraline (0.80) (online supplemental table 5). The heterogeneity parameter I2 was 0 (95% CI 0 to 0.9) and tau2 was 0 (online supplemental table 6). The Q value indicated within-design heterogeneity of 1.92 (p=0.17) and between-design inconsistency of 0.04 (p=0.84) (online supplemental table 7). Node-splitting analysis was performed to assess inconsistency. Only two comparisons had both direct and indirect evidence, with insignificant inconsistency: enzymolysed honeybee larvae (EHLP) versus EHLP and gabapentin and EHLP and gabapentin versus placebo from the same studies58 (online supplemental figure 5, online supplemental table 8). Nonetheless, given there is only one study reporting such evidence, the evidence level was rated as low in GRADE assessment results (online supplemental table 8). The funnel plot was generally symmetric, and Egger’s test showed no significant publication bias (p=0.83) (online supplemental figure 6).
(A) Network structure of changes in annoyance of tinnitus. (B) Forest plot of the changes in annoyance of tinnitus. The effect size <0 indicates the treatment was associated with higher improvement in annoyance of tinnitus than the placebo. ACA, acamprosate; EHLP, enzymolyzed honeybee larvae; GAB, gabapentin; HAN, hangekobokuto; ITDI, intratympanic dexamethasone injection; LAM, lamotrigine; NER, neramexane; PIR, piribedil; SER, sertraline.
NMA results for loudness
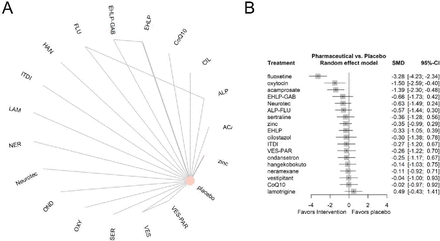
There are 27 studies reporting for loudness. Several studies were excluded from the analysis including Drew and Davies63 and Kucher et al,35 which did not disclose group-level data. Morgenstern and Biermann64 and Jalali et al65 provided the loudness in dB. The rest 23 studies also contain six disconnected studies.39 51–53 66 67 Thus, 17 studies with 19 interventions and 1357 patients were analysed (figure 3A). Six studies42 54–56 58 68 used 100 scale questionnaires and eight studies used 10 scale.11 40 48 59 61 69–71 Using the random effect model, the fluoxetine showed the largest reduction in loudness with an SMD of −3.28 (95% CI −4.23 to –2.34), followed by oxytocin (SMD: −1.5, 95% CI −2.59 to –0.4) and acamprosate (SMD: −1.39, 95% CI −2.3 to –0.48) (figure 3B). The detailed league table displayed all direct and indirect comparison results (online supplemental table 10). The SUCRA showed the top three treatments as fluoxetine, oxytocin and acamprosate with scores of 0.99, 0.87 and 0.87, respectively (online supplemental table 5). The heterogeneity parameter I2 was 0.63 (95% CI 0 to 0.89) with a τ2 of 0.15 (online supplemental table 6). The Q value revealed between-design inconsistency of 1.38 (p=0.24) and within-design heterogeneity of 3.98 (p=0.05) (online supplemental table 7). Similar to the annoyance results, the node-splitting analysis results retrieved one study58 with insignificant results and rated as low evidence (online supplemental figure 7, online supplemental table 8). Notably, the funnel plot showed an unsymmetric shape, and Egger’s test indicated a significant publication bias (p<0.05) (online supplemental figure 8).
(A) Network structure of changes in loudness of tinnitus. (B) Forest plot of the changes in loudness of tinnitus. The effect size <0 indicates the treatment was associated with higher improvement in loudness of tinnitus than the placebo. ACA, acamprosate; ALP, alprazolam; CoQ10, coenzyme Q10; CIL, cilostazol; EHLP, enzymolyzed honeybee larvae; GAB, gabapentin; FLU, fluoxetine; HAN, hangekobokuto; ITDI, intratympanic dexamethasone injection; LAM, lamotrigine; NER, neramexane; OND, ondansetron; OXY, oxytocin; PAR, paroxetine; SER, sertraline; VES, vestipitant.
Sensitivity analysis results
We conducted the leave-one-out analysis for the tinnitus severity outcome for testing the sensitivity of our results. We first calculated the SUCRA scores for every leave-one-out analysis result (online supplemental table 11) and then the proportion of each treatment in each ranking (online supplemental table 12). The proportion of SUCRA for the first-five and the last-five treatment rankings is shown in figure 4, with GB and vitamin the most prevalent best treatment (98%), following by caroverine the second (93%) and amitriptyline (47%) the third. Moreover, the studies reporting PP severity results were analysed for measuring the robustness of the results. A total of 25 studies, 26 interventions and 1634 patients were included, as depicted in online supplemental figure 9A. In terms of ranking, GB with vitamin emerged as the top choices similar to the whole analysis (online supplemental figure 9B), demonstrating a significant reduction in severity (SMD: −3.64, 95% CI −4.48 to –2.80) under a fixed effect model. The league table and SUCRA results are available in online supplemental tables 13 and 5. Regarding heterogeneity and inconsistency, the I2, tau2, and Q statistics were 0 (95% CI: 0, 0.85), 0, 2.09 (p = 0.55), respectively2 (online supplemental tables 6 and 7).
The proportion of each treatment rankings among the circumstances when excluding one study each time using leave one out analysis. The treatment with the most percentage was shown above each column. EHLP, enzymolyzed honeybee larvae; GB, Ginkgo biloba; ITDI: intratympanic dexamethasone injection; Vit, vitamin.
Subgroup analysis results
As shown in online supplemental table 2, we classified the RCTs into chronic or acute based on either a clear implication within the original paper or whether actual duration of tinnitus is longer than 6 months or not.1 21 There were 29 studies identified and the Meeus et al,51 Prochazkova et al52 and Han et al53 could not be connected. A total of 26 studies, 28 interventions and 2129 patients were included as shown in online supplemental figure 10A. The gabapentin with lidocaine ranked the first (online supplemental figure 10B) and showed significant severity decrease compared with the placebo (SMD: −0.84, 95 CI −1.36 to –0.31) in a fixed effect model. The league table and SUCRA results are provided in online supplemental tables 14 and 5. The I2 τ2 and Q statistics for within-design heterogeneity and between-design inconsistency were 0.22 (95% CI: 0, 0.92), 0.02, 0.12 (p=0.73), and 2.45 (p=0.12), respectively (online supplemental tables 6 and 7). We also separated the studies based on measurement scales (THI used) and ROB (only low ROB studies). There are 31 studies using THI identified with 32 interventions, and 2371 patients included, as shown in online supplemental figure 11A. Caroverine exhibited a significant decrease in severity (SMD: −1.96, 95% CI: −2.74 to –1.18) in a fixed effect model (online supplemental figure 11B, online supplemental table 15). Caroverine (SUCRA: 0.99) and gabapentin combined with lidocaine (SUCRA: 0.88) ranked the first and the second as SUCRA results shown in online supplemental table 5. The I2, tau2 and total Q statistics were 0.1 (95% CI: 0 to 0.77), 0.01, and 5.58 (p=0.35), respectively (online supplemental tables 6 and 7). For the studies with low ROB, Prochazkova et al could not be connected and 8 studies with 10 treatments were analysed (online supplemental figure 12). The results showed acamprosate as the most effective compared with placebo (SMD: −0.81, 95% CI: −1.72 to 0.11) (online supplemental table 16). The Q statistics together with I2 and tau22 could not be measured since the scarcity number of connected treatment other than connecting with placebo (online supplemental tables 6 and 7).
Discussion
We comprehensively investigated the effects of different pharmaceutical treatments for subjective tinnitus as decreasing the severity, annoyance and loudness. Interestingly, the GB with vitamin and caroverine treatment showed an optimal effect while with low evidence of certainty. After removing studies with high ROB or certain concerns, the subgroup analysis changed greatly as the acamprosate, a glutamate antagonist and GABA agonist, turned to be the first-ranked treatment compared with placebo. Additionally, the acamprosate accomplished a favourable treatment effect as ranked the first and the third treatment in the annoyance and loudness outcomes. This encouraging effect could be correlated with one pathophysiology models of tinnitus, which suggests the tinnitus originates from imbalance between excitatory and inhibitory neurotransmitter systems in the auditory pathway, where GABA plays as an essential inhibitory neurotransmitter.72 73 Elevated spontaneous neural activity likely arises from an impaired GABA synaptic transmission or an increase in the release of excitatory neurotransmitters like glutamate, leading to the occurrence of tinnitus.74 Except acamprosate, a series of other GABAkines drugs have been included in our study, including gabapentin,49 58 75 76 clonazepam53 and melatonin.36 67 77 However, clonazepam and melatonin were ranked lower than 10th regarding the severity and unavailable for annoyance and loudness. Notably, the combination of gabapentin with EHLP achieved within the top five in decreasing the annoyance and loudness while the single use of gabapentin retrieved lower rankings. The mechanism of gabapentin in treating tinnitus is still in vague49 while for EHLP with gabapentin, given there is only one study reporting related result.58 The author explained that it could be associated with the antioxidant effects in the EHLP but no reports have been published on the effects of bee larvae on inner ear sensory cells, which should be examined in the future studies.58 Although, the neurochemical alterations and specific anatomical and physiological associations with GABA synapses in tinnitus suggest that enhancing GABAkines inhibition could potentially improve subjective tinnitus symptoms, the evidence of the effectiveness of GABAkines in treating subjective tinnitus is not yet surely convincing. Future studies with longer follow-up duration and novel design are needed to verify further.
Several herbal medicines were assessed including the GB, and hangekobokuto.56 GB is a commonly used herbal supplement for tinnitus78 but its efficacy in treating tinnitus remains controversial. Interestingly, in our results, seven studies used the GB intervention,38 43 47 52 53 63 64 which showed significant THI decrease compared with the placebo group. GB has been shown to have several central nervous system effects that may increase neurotransmitter levels and neuronal plasticity, as demonstrated in animal and human studies.79 Its potential mechanisms for treating tinnitus include preventing free-radical damage to the cochlea, improving cochlear microcirculation, increasing blood flow, and alleviating ageing-associated degeneration, ultimately improving tinnitus symptoms.80 However, a recent review by Bassel et al highlighted the limited and conflicting evidence for the use of GB in vertigo and/or tinnitus, citing variations in evidence quality, poor reporting standard and different outcome measures as contributing factors.81 It’s important for clinicians to be aware of the increased risk of bleeding associated with GB, especially in patients with underlying clotting disorders.78
There are increasing investigation interests focusing on the treatment of tinnitus as the growing number of meta-analyses recently. In 2021, Chen et al82 analysed 36 RCTs (2761 participants, from inception to 5 April 2021) on pharmacologic treatments for primary tinnitus. The primary outcome was tinnitus severity, finding brain-acting drugs (eg, amitriptyline) and anti-inflammatory agents superior to placebo but with no impact on quality of life. Nevertheless, the authors concluded that given that some intervention comparisons were grounded in a limited number of RCTs and the rating scales for tinnitus severity and other subjective symptoms exhibited significant variability, clinicians should exercise caution when selecting specific pharmacologic treatments. Our study focused on the subjective tinnitus and updated the search to 2025. Notably, similar results were observed as the central nervous system treatments and anti-inflammatory agents were ranked higher (ie, amitriptyline ranked the fourth in decreasing the tinnitus severity). The heterogeneity within designs and between studies and the scarcity of repeated comparisons should also be acknowledged. As the results, Chen et al and our results both showed low or very low quality of evidence from the perspective of GRADE assessment. In two other recent published articles in 2024, Lu et al83 examined 22 RCT studies (2354 patients, from inception to December 31 2022) on non-invasive therapies for chronic tinnitus. They organised their results based on outcome indicators like THI and VAS, concluding that acoustic therapy combined with cognitive behavioural therapy was most effective for reducing THI scores. Another article by Waters et al84 included 23 studies (1626 participants, from inception to October 2022), demonstrating placebo improvements in both THI and loudness but inferior than non-placebo interventions. Novel treatments were evaluated in meta-analysis as well. In 2025, a meta-analysis on scalp acupuncture included 20 studies (1430 participants, from inception to April 2024), reporting significant clinical efficacy and reduced tinnitus severity compared with controls, though with moderate heterogeneity.85
The limitations of the current NMA primarily stem from heterogeneity among studies and methodological challenges. First, heterogeneity arises from variations in study design, treatment protocol, outcome assessment scales and the statistical analysis method, which can undermine the validity of pooled results if not adequately addressed through subgroup or sensitivity analyses. The extensive number of treatments and pairwise comparisons included in this analysis increases the possibility of driving statistically significant findings, which underscores the need for cautious interpretation. Moreover, although publication bias was observed only by one analysis in our study, the exaggerating effect by dominating studies with small sample sizes in the current analysis should be underlined. Additionally, the choice between fixed-effect and random-effects models introduces variability as well as random-effects models may overemphasise smaller studies in heterogeneous datasets, while fixed-effect models assume unwarranted homogeneity. Furthermore, clinical trials without proper placebo group resulted in several disconnected treatment arms, which could not be included in the analysis and potentially undermined the scope of the current study. Addressing these limitations requires rigorous methodology, transparency, and cautious application of findings.
Conclusion
In summary, our findings revealed the potential of antioxidant and GABA agonists therapy might be promising choices for subjective tinnitus. Nonetheless, the current study is limited by the small number of repeated comparisons, the wide range of questionnaire differences and discrepancies in clinical settings. Future rigorously designed researches should include larger sample sizes and longer follow-up durations for more systematic investigations of subjective tinnitus treatments.
Supplemental material
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
References
Footnotes
Contributors PL: data curation, formal analysis, visualisation, writing—original draft. CC: data curation, validation, investigation. YW: conceptualisation; methodology, project administration. SS: conceptualisation, funding acquisition, supervision; writing—review and editing. SS is responsible for the overall content as the guarantor. PL: data curation, formal analysis, visualisation, writing—original draft. CC: data curation, validation, investigation. YW: conceptualisation; methodology, project administration. SS: conceptualisation, funding acquisition, supervision; writing—review and editing. SS is responsible for the overall content as the guarantor.
Funding This study was sponsored by the Ministry of Science and Technology (2023YFC2508402); National Natural Science Foundation of China (Number 82371146, 82192862) and Research Projects of Shanghai Municipal Health Committee (2022XD059).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer-reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.