Article Text
Abstract
Introduction Postoperative pain management is a major concern for patients undergoing distal radius open reduction internal fixation (ORIF). Inadequate pain control negatively impacts patient’s satisfaction and may increase opioid use. Topical tranexamic acid (TXA) has been demonstrated as an effective intervention that reduced acute postoperative pain in total knee arthroplasty. There is no study evaluating the effects of TXA on acute postoperative pain for distal radius ORIF. This study aims to evaluate the effect of topical TXA administration during isolated distal radius ORIF on early postoperative pain.
Methods and analysis The effect of topical TRanexamic Acid versus placebo on acute postoperative pain following Distal Radius fracture fixation (TRADR) study is a randomised controlled double-blinded trial that will enrol 90 patients, 18 years of age or older, undergoing volar open reduction internal fixation. Patients will be randomly assigned to topical TXA versus topical saline (placebo) in a 1:1 ratio. The surgeon at the time of surgical closure after standard distal radius fixation will apply either 1 g of topical TXA (100 mg/mL; treatment group) or 10 mL of saline (control group) to the wound and let it sit for 5 min. Surgeons, patients, and outcome assessors will be blinded to the treatment group. The primary outcome is acute postsurgical pain as measured by the visual analogue scale (VAS). Pain outcomes will be between postoperative days 0 to 7, and at 2 and 6 weeks postsurgery. The secondary outcomes include opioid usage, unscheduled emergency visits, wrist swelling and adverse events.
Ethics and dissemination This study was approved by the University Health Network Research Ethics Board (REB 23–5708). The results of this trial will be disseminated through peer-reviewed journals and presented at related conferences. The principal investigator will communicate the results with patients who have indicated an interest in knowing the results.
Trial registration number Clinicaltrials.gov NCT06384456, April 26, 2024; Pre-enrolment.
Protocol version Version 2.0: August 26, 2024.
- ORTHOPAEDIC & TRAUMA SURGERY
- Hand & wrist
- PAIN MANAGEMENT
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study uses a prospective, randomised and blinded design to evaluate the effects of topical tranexamic acid on acute postoperative pain after distal radius open reduction internal fixation.
This study follows stringent methodological approaches to reduce bias, including the use of a web-based central randomization system (Research Electronic Data Capture), blinding of investigators, patients and outcome assessors, align post-op visits with typical standard of care visits to minimise the loss to follow-up and accuracy of outcome assessments, and document all possible adverse events.
This study will only collect data up to 6 weeks postoperatively, which may not account for subtle differences that may occur at longer follow-up time points.
Introduction
Distal radius fractures (DRF) are the most common hand or forearm fracture and represent 10% to 25% of all fractures.1–3 Although some fractures may be managed conservatively, surgical fixation can allow patients to return to independent daily activities earlier.4 This surgery, however, can be associated with pain in the early postoperative period. One of the dominant predictors of a patient’s perception of recovery after surgical fixation is the degree of pain.5 Current pain management protocols emphasise multimodal pain control to reduce opioid consumption.6 Optimising perioperative preventive pain strategies (both in surgical technique and adjunct therapy) could provide greater improvements in patient’s function and satisfaction, decrease postoperative opioid use and ultimately reduce the development of chronic postoperative pain syndromes.
Tranexamic acid (TXA) is a well-established medication that has been used extensively in orthopaedic surgery, for the purposes of decreasing intra- and postoperative bleeding, transfusion rates and, more recently, has been utilised as perioperative preventative adjuncts for reducing pain.7–9 TXA inhibits the activation of plasminogen to plasmin, stabilising the fibrin clot.10 Inflammation can be attenuated by modulating levels of plasminogen and plasmin, as they play an important role in the balance of inflammation.10 A recent systematic review10 on the impact of TXA on inflammation found multiple studies that assessed TXA compared with control and found that TXA administration led to decreased interleukin-6 (IL-6) and C-reactive protein levels.10 Thereby modulating inflammation and subsequent activation of nociceptors, this may impact a patient’s subjective pain response.11
TXA can be given intravenously (IV) or given as a topical solution that bathes the wound prior to closure. There have been multiple studies in different orthopaedic specialties that have demonstrated that IV TXA leads to decreased pain, along with reduced blood loss and joint swelling.7 9 12 Although there has been less emphasis on topical TXA in the literature, multiple studies have found comparable reductions in pain when using topical TXA compared with IV.13 14 In particular, a study assessing TXA use in unilateral total knee arthroplasty found decreased rates of pain when TXA-soaked gauze and intraoperative spraying were used compared with IV TXA.14 Although both groups had TXA injected into the joint at the end of the case, the intraoperative spraying may have contributed to the significant difference in pain at postoperative Days 1 and 3.14 At a minimum, the combination of additional local TXA was at least comparable in reducing pain when compared with IV administration, when both groups had local TXA injected.14
There is limited research directly assessing the impact topical TXA has on pain (when compared with placebo), but a previous study comparing topical TXA to placebo in arthroscopic ACLR found statistically significant reductions in pain scores on postoperative Day 3 and at Week 4.8 Additionally, a previous study demonstrated that in hip arthroplasty, there were decreased inflammatory markers (CRP and IL-6) on postoperative Days 1, 3, 7 and 10.15 Therefore, topical TXA appears to decrease local inflammation, which may lead to decreased levels of pain, and is surgeon preference at our institution for distal radius fracture fixation.
Despite the usage of topical TXA in multiple orthopaedic specialties and procedures, there is a lack of research on the use of TXA in DRF fixation as a potential perioperative intervention to decrease pain.8 16–18 In particular, the use of the topical TXA in this setting has not been previously studied. Because of the high incidence of distal radius fractures and relatively high prevalence of patients with acute pain following the surgery, assessing the effect of TXA as a potential perioperative pain intervention could have a considerable impact on patients’ quality of care.19 20 Furthermore, given the widespread use of opioids despite the efforts to optimise postoperative pain control, pain reduction could decrease the development of chronic pain and misuse of opioids as well.
Therefore, this study aims to evaluate the effect of topical TXA administration during isolated distal radius open reduction and internal fixation (ORIF) on early postoperative pain. Our hypothesis is that topical TXA will reduce early postoperative pain, improve patient-reported outcome scores and reduce opioid use.
Methods
Trial design
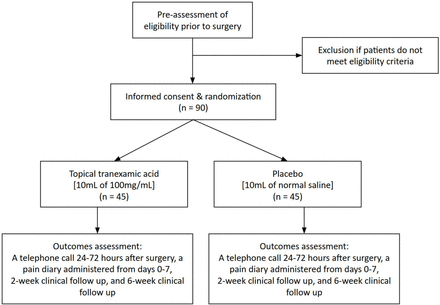
We propose a randomized, parallel group, double-blinded, placebo-controlled trial of the effect of topical TXA administration into the surgical wound prior to closure on acute postsurgical pain in patients with DRF treated with ORIF (figure 1). The protocol was created following the Standard Protocol Items: Recommendations for Interventional Trial (SPIRIT) reporting guidelines.21
The TRADR study design.
Trial registration
The TRADR study was registered on ClinicalTrials.gov NCT06384456.
Participants and setting
Patients who are scheduled for ORIF of DRF will be prescreened, and full eligibility will be determined during the primary appointment with an orthopaedic surgeon. This trial will be conducted at Toronto Western Hospital (TWH) and will only involve the primary investigators and coinvestigators of this study. All surgeons are fellowship trained and have performed a minimum of 10 distal radius ORIF operations as a primary surgeon in the past year. The starting date is anticipated to be May 2025, and the anticipated end date is August 2027.
Eligibility criteria
Broad eligibility criteria will be used to increase the generalisability of the trial’s results. Patients undergoing ORIF (Volar approach) for operative management of a DRF aged 18 years or older will be eligible for this study. All fracture mechanisms (high or low energy), young or elderly patients, and associated distal ulna fractures (treated non-operatively) will be included. Patients who are willing to provide written informed consent, have the capacity to consent as deemed by clinical judgement and have English-language skills to complete outcome measures will be eligible.
In accordance with Health Canada recommendations, patients will be excluded if any of the following criteria are met: (1) revision surgery or any additional operative management of ipsilateral wrist injury, (2) DRF treated with a dorsal approach, (3) known history of lymphedema or lymph node dissection in the operative extremity, (4) known chronic pain conditions, fibromyalgia or polymyalgia rheumatica, (5) current user of opioids and/or on chronic opioid use, (6) known allergic reaction to TXA, (7) anticoagulant use not stopped in time for surgery as per Thrombosis Canada guidelines (ie, warfarin, acetylsalicylic acid, direct oral anticoagulants, etc.), (8) previous thrombotic stroke or thromboembolic disorders (ie, known previous deep vein thrombosis, pulmonary embolism or clotting disorders), (9) current pregnancy or breastfeeding, (10) previous neurologic injury causing paralysis of affected shoulder/arm, (11) severe cardiorespiratory disease (ie, American Society of Anesthesiologists Grade IV or higher), (12) current contraceptive pill use or hormone replacement therapy, (13) patients taking medications including: hydrochlorothiazide, desmopressin, sulbactam-ampicillin, carbazochrome, ranitidine or nitroglycerin or (14) intolerance/allergy to hydromorphone or codeine.
Randomization and blinding
Informed consent will be obtained by research personnel, residents and staff surgeons (online supplemental appendix A). After informed consent has been acquired, eligible patients will be randomized to either intervention or placebo. A statistician who is not involved in clinical procedure will generate a random sequence table using Stata software to create a 1:1 allocation scheme with permuted blocks with random block sizes. To ensure the concealment, we will upload the randomization list on Research Electronic Data Capture (RedCap).22 The random sequence will be concealed from all study personnel (except the statistician and study REDCap programmers), including the investigators, site staff and participants. Following tourniquet deflation, the blinded surgeon will pour the prepared solution into the wound directly from the medicine cup. Blinded study personnel will not have access to the list identifying which package number belongs to each group. Study drugs will be stored in a locked place at the TWH pharmacy department and will be managed only by the open-labelled pharmacists. The steering committee will audit the blinding and adherence to the protocol annually and as needed. An unblinding procedure will be made available in case of emergencies or reported adverse events.
Supplemental material
Trial interventions
Eligible patients randomized to the intervention will receive 100 mg/mL × 10 mL vial of TXA (Sandoz, Canada) in addition to the standard care. Patients randomized to the control group will receive 0.9% sodium chloride injection USP × 10 mL vial in addition to the standard care. TXA is a colourless fluid and is not distinguishable from saline to the naked eye.
Standardised preoperative nerve block
All patients will receive a brachial plexus block for surgical anaesthesia. Exact location will be based on anaesthesiologist preferences between supraclavicular, infraclavicular or axillary. The local anaesthetic that will be administered will be a 1:1 mixture of 2% lidocaine and 0.5% bupivacaine. IV dexamethasone will not be given to patients. Standard preoperative IV antibiotics will be given.
Standardised distal radius ORIF
Surgery will be performed in the supine position with an arm board. A pneumatic tourniquet will be used. An extended flexor carpi radialis (FCR) approach will be utilised. The FCR tendon will be palpated, and a 10-cm incision will be made over it. Subcutaneous tissue will be dissected down to the FCR tendon. A longitudinal incision will be made centred over the FCR tendon. The tendon will be retracted ulnarly. A second longitudinal incision will be made through the floor of the FCR sheath. Flexor pollicis longus will be identified and partially mobilised off the radius as needed to retract ulnarly and expose the pronator quadratus (PQ). PQ will be cut distally and radially and retracted ulnarly to expose the surface of the volar aspect of the distal radius. Standard fracture care will be undertaken to properly reduce the fracture fragments and align anatomically. Intraoperative fluoroscopy will be used for the reduction and fixation. Fixation with Synthes distal radius variable angle locking compression plates and locking/non-locking screws as per surgeon discretion. Partial brachioradialis release will be undertaken as needed for fracture reduction. After fracture fixation, the pronator quadratus will be repaired and the tourniquet will be deflated. Meticulous haemostasis will be attempted prior to the application of topical TXA or saline solution (depending on randomized treatment arm). The surgeon will pour the unmarked solution into the surgical wound, and the unmarked solution will be allowed to sit in the wound for 5 min. 5 min has been selected as previous trials investigating the use of topical TXA, primarily in the setting of arthroplasty, allowed the solution to sit in the wound for 5 min prior to suctioning and closure.17 18 The solution will be suctioned, and meticulous haemostasis will be attempted again to stop any concerning bleeding. The wound will be closed in a typical manner. A standard dressing and plaster splint will be applied to the incision. No local anaesthetic will be applied to the skin after closure.
Postoperative routine
The postoperative therapy regimen will be the same for each group. All patients will be kept in a plaster splint for 2 weeks until their first in-person follow-up, with instructions to not bear weight on the affected limb. During the first 2 weeks, the patients will be encouraged to move their fingers but otherwise not bear weight. At 2 weeks postsurgery, patients will be placed in a removable splint with instructions to not bear weight until 6 weeks follow-up. A standardised, detailed, postoperative physical therapy protocol will be provided for all patients at 2 weeks.
Outcomes assessment
The primary outcome of this study is to determine the effect of TXA versus placebo on acute postoperative pain at 72 hours postoperatively. It will be assessed with VAS on a 0 to 10 cm scale, with a higher score indicating worse pain. This will be collected using a patient-completed daily pain diary for the first 7 days and a one-time phone call (between 24 to 72 hours postoperation). The primary method of VAS assessment will be through the pain diary. The phone call will be used as a backup VAS score, reminding patients to complete their pain diary and answer any questions they have about the process to improve patient participation and retention.
The secondary outcome measures will be to determine the effect of topical TXA versus placebo on persistent pain at follow-up visits. Patient-reported functional outcomes will also be assessed using the Disabilities of the Arm, Shoulder and Hand questionnaire (DASH) score 0 to 100,23 with higher scores indicating more disability. DASH score will be assessed in person during the preoperative assessment (indicating preinjury DASH scores), and then again in person at the 6-week follow-up. We will also assess the unscheduled hand-related clinic/emergency unit visits or any issues or events related to the condition that might lead participants to visit the emergency room, be seen by a doctor or undergo any intervention more frequently than the usual visits. All serious and non-serious adverse events will be documented on the study-specific case report forms. An independent medical monitor will also review each adverse event to determine its relatedness to the surgical treatment. All serious adverse events will be followed to the resolution or stabilisation of signs and symptoms. If concerns regarding increased adverse events with treatment arise, the steering committee will be involved in reassessing the data and will make the final decision if trial termination is required.
Physical exam measurements at each follow-up visit (2 and 6 weeks) will be used to evaluate any wrist swelling, bruising, wound breakdown and infection. Swelling will be determined by measuring the circumference of each wrist and comparing the percentage difference in sizes recorded. To ensure reliability and consistency, a standardised technique will be employed to measure the wrist circumference with the distal edge of the width of the measuring tape placed at the distal wrist crease as previously described.24 All assessors will be trained on how to appropriately measure wrist circumference to improve reliability. Bruising will be reported in a dichotomous (yes/no) method if there is any discolouration of the skin representative of internal bleeding. Wound breakdown will be reported in a dichotomous (yes/no) method if there is any loss of opposition of skin edges at the incision. Infection will be reported in a dichotomous (yes/no) method if there are any signs of superficial or deep infection based on the assessor’s clinical judgement (purulence, erythema, rubor, fevers, worsening pain, blood work, etc.).
Study follow-up times
Details of all outcomes and the follow-up visits are presented in table 1 as per SPIRIT guidelines. An electronic case report form (CRF) will be created in the Redcap system, and a trained research staff will directly enter data from the trial site. Trained assessors, blinded to the intervention, will also collect data on the outcomes at the follow-up time points: preoperative clinic visit, between 24 and 72 hours postoperative (by phone call), 0 to 7 days (diary) and at 2 and 6 weeks postsurgery (in-person visit). Patients will have a full assessment at their preoperative clinic visit, will complete a DASH questionnaire and will be trained on how to complete the daily pain diary. The provided pain diary will be completed by patients in the morning and the night for the first 7 days postoperation (online supplemental appendix B). The 24- to 72-hour postoperative phone call will be completed to ensure patients are completing their pain diary, answer any questions they may have about the study and obtain a pain level at that time. This will be done to maximise retention. The pain diary will determine the overall level of pain they are experiencing (since they last filled the diary), their current usage of opioids and their current usage of other pain medication (acetaminophen). At the 2-week standard visit, pain diaries will be collected, the cast will be changed, sutures will be removed and a clinical assessment will be performed. At this time, patients’ pain levels, opioid use, adverse events and unplanned events since surgery will be tracked, and a thorough physical examination will be performed. At the 6-week standard visit, the cast will be removed and hand therapy will be initiated. Patients will complete a DASH questionnaire at that time. All continued opioid use will be tracked, along with any interim hospital presentations, adverse events or unplanned hand-related procedures (ie, premature cast changes, carpal tunnel releases, haematoma evacuation, etc.). A repeat physical examination will be completed at that time.
Supplemental material
The schedule of enrolment, interventions and assessments
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting or dissemination plans of our research.
Maximising patient retention
To ensure patient retention and data completion, a postoperative phone call between 48 and 72 hours postoperatively will be made to the patients to answer any questions, remind them to fill out their diary and obtain a pain measurement. Also, the follow-up schedule will be aligned with the typical standard of care visits.
Statistical plan
Sample size
A sample size calculation demonstrated that 90 patients (45 in each group) would be required to identify a 1-cm difference in the visual analogue scale (VAS) pain score at 48 hours post-op based on a minimum clinically important difference.25 Sample size is based on the two-sided test, alpha level of 0.05 and power of 90% and includes an adjustment of 12% for patients who may withdraw or become lost to follow-up. To maximise recruitment, information about the trial will be disseminated throughout our institution, including the Department of Orthopaedics, the Emergency Department, and our affiliated hospitals at Toronto General Hospital and Mount Sinai Hospital.
Analysis plan
The analysis and reporting of results will follow the CONSORT guidelines for reporting of randomized controlled trials. Statistical analysis will be performed by an independent statistician not involved in the treatment. The primary analysis will include patients in the groups to which they have been randomly assigned (intention to treat principle) with performing no imputation. The process of participant enrolment throughout the study will be summarised using a flow diagram. Participant demographics, medical history, surgical details and perioperative details will be summarised by treatment group using descriptive summary measures: expressed as mean and SD or median and IQR for continuous variables, depending on the distribution, and number and percent for categorical variables.
The analysis will compare intervention groups (TXA vs placebo) on their mean change in VAS pain, opioid use and other clinical outcomes between baseline and each follow-up using a multivariable linear mixed model. Subjects will be considered as random effects, treatment group and visit number as fixed effects. Independent variables will be the intervention groups, and baseline value of pain, age, sex and fracture pattern (intra-articular vs extra-articular) will be included as covariates in the regression model. The estimated difference in mean change from baseline to each follow-up and the corresponding 95% CI will be presented. For the binary outcomes (opioid use, adverse events and unplanned hand-related procedure), the logistic regression will be used, and the odds ratio and corresponding 95% CI will be reported. All tests will be two-sided, and the alpha level will be 0.05. We will use Stata (StataCorp., Release 15.1. College Station, TX, USA) for all analyses.
Data management & confidentiality
Patients’ information will be kept confidential through the study, and all study-related data will be entered into REDCap, a secure, web-based application designed exclusively to support data capture for research studies.22 26 REDCap is licensed free of charge by the Research Institute at University Health Network (UHN), and the application and data are housed on servers provided by UHN. There will be a dedicated research assistant who will be responsible for data entry in the REDCap database. This research assistant will be responsible for data transcription and entry, data verification and running queries as requested by the investigation team. All CRFs will be identified only by a coded patient’s number and initials. All records that contain patient names or other identifying information will be stored separately on password-protected computers in the trial site at TWH. All members of the team for inputting or analysing information and data will have secure password-protected access to the data for the duration of the trial. The primary investigators will have access to the trial data for up to 10 years.
All of the following severe adverse events will be thoroughly investigated for consideration of termination: postoperative death, stroke, myocardial infarction, seizure, permanent vision changes, allergic reaction, anaphylaxis or pulmonary embolism. Specifically, if there are more than three patients with a postoperative death, stroke, myocardial infarction, seizure or pulmonary embolism in the TXA group than those in the placebo group, a decision of termination will be strongly considered as these outcomes are severe and unexpected complications of both distal radius fracture fixation as well as possible topical TXA administration. Additionally, if there are two or more patients with permanent vision changes, allergic reaction or anaphylaxis in the TXA group than those in the placebo group, a decision of termination will be strongly considered. This decision will be made by the members of the steering committee and data monitoring committee.
Subgroup analysis
We will perform an exploratory subgroup analysis based on intra-articular fractures involvement compared with solely extra-articular fractures, as visible on X-ray or CT.
Discussion
The TRADR study will assess whether topical TXA administration in distal radius fractures treated with ORIF will lead to reduced early postoperative pain. There have been previous studies that suggested the efficacy of topical TXA in diminishing postoperative pain and swelling across other orthopaedic procedures;16 27 however, this has not been studied for the treatment of early pain in DR ORIF. Therefore, this study will provide key insights into the potential topical TXA may have in the treatment process of a very common and important orthopaedic procedure.
There are only limited methods to treat pain in the acute setting, whereby most postoperative pain (once nerve blocks have worn off and patients are weaned off intravenous pain medication if needed) is effectively treated with oral pain medication.28 Despite recent advocacy for multimodal oral pain management approaches, the predominant method still involves opioid medication alongside acetaminophen. Some surgeons remain cautious about incorporating alternatives like NSAIDs due to concerns regarding potential delays in bone healing.29 The application of TXA is known to reduce surgical site bleeding, potentially leading to a reduction in swelling, inflammation and decreasing pain after surgery.7 Additionally, decreasing the pain could impact opioid use.
Furthermore, the issue of postoperative pain can impact a patient’s overall experience of surgery. In total knee literature, patients who experience more pain have been shown to have worse outcomes.30 In distal radius surgical literature, there is evidence to suggest that worse preoperative pain is associated with a greater risk of chronic pain.31 It has been hypothesised that the development of complex regional pain syndrome (CRPS) is caused in part by ongoing and severe nociceptor response leading to central hyperexcitability.32 Therefore, by reducing the nociceptor response by reducing swelling and inflammation by applying topical TXA, this could reduce the development of CRPS, although this is yet to be proven.
The hand and wrist are particularly at risk for postoperative swelling.33 It is commonly advised to reduce swelling by elevating the affected limb above the level of the heart to enhance venous return. However, due to the use of splints postsurgery, options like icing or gentle movement of the wrist/hand are often restricted. Excessive swelling can clinically result in a tighter fitting splint, exacerbating pain and potentially causing paresthesias. Consequently, patients may need to revisit the clinic or emergency department for a cast change. Evidence from shoulder and elbow literature suggests that topical TXA can reduce swelling,16 27 and if it has a similar effect in the wrist, it could potentially decrease the need for cast changes due to swelling. Oftentimes, orthopaedic research is limited by the inability to blind the surgeon, as one cannot both perform and be blinded on the exact surgery being performed. Topical TXA is an excellent option for a double-blinded randomized controlled trial as it is an easy treatment to blind. It is a colourless fluid, indistinguishable in colour or consistency from saline. The surgeon will be entirely blinded to the application of either treatment or placebo, further reducing the risk of bias. Topical TXA is an important adjunct to explore in orthopaedic surgery. Even more importantly, it has been found to be safe to administer locally without increasing the risk of developing venous thromboembolic events.34 As it has demonstrated promising results in other surgical procedures, it should be explored as an important adjunct for the surgical treatment of distal radius fractures that could make important clinical improvements for patients.
Limitations
There are a few limitations for our study. The primary limitation is the challenges around obtaining accurate pain levels and opioids used by each patient. The primary method for obtaining early postoperative pain values is through the use of an at-home diary. This can be subject to issues with completion, recall bias and errors in data collection. To mitigate these issues, patients will receive training on how to complete the diary as well as receive a phone call 48–72 hours after surgery to help answer any questions, get a recorded pain value and remind the patients to complete them. Another limitation is the timeframe for which outcomes will be followed. Given the primary aim of the study is early postoperative pain (first few days to week after surgery), data will only be collected up to 6 weeks postoperatively. This timeframe may not be long enough to determine subtle differences in outcome that may unexpectedly occur at longer follow-up time points. Also, by setting restrictive eligibility criteria to minimise interperson heterogeneity and enhance patient safety, the trial’s ability to be fully pragmatic may be limited. Lastly, given the possible heterogeneity of fracture mechanisms, intra-articular compared with extra-articular fractures and patient age groups (young compared with elderly), subgroup analysis may be underpowered and limited.
Ethics and dissemination
This study obtained Health Canada approval through a No Objection Letter (NOL): #287 930. The protocol of the TRADR study was reviewed and approved by the UHN REB (23–5708). Any amendments or updates to the protocol will be updated through the UHN research ethics committee. All documentation will be submitted to this committee as well as updating our registration on ClinicalTrials.gov. Results from the study will be submitted for publication regardless of whether or not there are significant findings. Access to our trial documentation, including data collection forms or trial’s data for secondary publications, will be made available by request and/or appendices accompanying a possible publication. Every attempt will be made to ensure that the amount of time between the completion of data collection and release of study findings is minimised. We will report required results on Clinicaltrials.gov or other applicable clinical trials registry.
Individual Participant Data (IDP) sharing statement
This study plans on sharing deidentified individual clinical trial participant-level data (IDP) in the event that a patient has a particularly unexpected or eventful postoperative course, in order to better characterise all outcomes this study intends to assess.
Ethics statements
Patient consent for publication
Acknowledgments
Independent Data Monitoring Committee: Dr Kevin Zuo, Plastic Surgeon, Plastic Surgeon – Sprott Department of Surgery, Toronto Western Hospital - Independent member. Dr Timothy Leroux, Orthopaedic Surgeon, Orthopaedic Surgeon – University Health Network, Toronto Western Hospital - Independent member. Mr. Daniel Antflek, BSc, Clinical Research Coordinator – Department of Orthopaedics, University Health Network, University of Toronto - Independent member.
References
Footnotes
Contributors Writing committee: AN, DLL and AA. Conception and design of the study: AC, RP, DLL and AN. Critically revised the manuscript: AC, RP, JP, KZ, HB, CV and EC. Guarantor: RP. All authors read and approved the final manuscript.
Funding This work was supported by the Canadian Orthopaedic Research Legacy (CORL) grant (# N/A) via the Canadian Orthopaedic Foundation, and the Arthrex WeCan Grant (#2024-003-002) via the Wrist and Elbow Society of Canada. The funding sources do not participate in designing or conducting the trial, collecting, managing, analysing or interpreting data, nor in preparing, reviewing or approving the manuscript for submission.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.