Article Text
Abstract
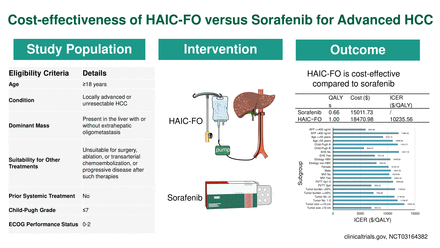
Objectives This post hoc study aimed to evaluate the cost-effectiveness of hepatic artery infusion chemotherapy (HAIC) with fluorouracil, leucovorin and oxaliplatin (HAIC-FO) compared with sorafenib in patients with advanced hepatocellular carcinoma (HCC). The analysis was conducted from the perspective of Chinese payers.
Design A cost-effectiveness analysis was performed using a Markov model derived from data obtained in the FOHAIC-1 trial (phase 3 randomised controlled trial; conducted 2017–2020).
Setting The study was conducted in tertiary care centres in China.
Participants The study included advanced HCC patients enrolled in the FOHAIC-1 trial. Inclusion criteria followed the trial protocols, with patients stratified by disease severity (including the presence of Vp4 portal vein tumour thrombus (PVTT) and high tumour burden).
Interventions HAIC-FO (fluorouracil, leucovorin and oxaliplatin) was compared with sorafenib for cost and health outcomes.
Primary outcome measure The primary outcome was the incremental cost-effectiveness ratio (ICER), calculated as the additional cost per quality-adjusted life year (QALY) gained.
Results Sorafenib yielded 0.66 QALYs at a cost of $15 011.73, whereas HAIC-FO yielded 1.00 QALY at a cost of $18 470.98. The ICER of HAIC-FO compared with sorafenib was $10 235.56 per QALY, which was below the willingness-to-pay (WTP) threshold of $30 492.00 per QALY. Sensitivity analyses confirmed that HAIC-FO remained cost-effective across variable assumptions, with probabilistic sensitivity analysis showing a 99.9% probability of cost-effectiveness at the WTP threshold. Subgroup analyses demonstrated more favourable ICERs for patients with Vp4 PVTT ($7003.33 per QALY) and those with high tumour burden ($7382.86 per QALY).
Conclusions HAIC-FO is a more cost-effective treatment for advanced HCC than sorafenib from the Chinese payer’s perspective, particularly in patients with Vp4 PVTT and/or high tumour burden. Further research is needed to explore long-term economic implications and real-world effectiveness data.
Trial registration number NCT03164382.
- Health economics
- Hepatobiliary tumours
- Interventional radiology
Data availability statement
Data are available in a public, open access repository. The datasets generated or analysed during the study are available in the Research Data Deposit repository (https://www.researchdata.org.cn/Search.aspx?k=RDDA2021002021).
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
The study used a Markov model based on data from the FOHAIC-1 phase 3 trial, providing robust comparative data between HAIC-FO and sorafenib.
The incremental cost-effectiveness ratio for HAIC-FO was well below the willingness-to-pay threshold, confirming its cost-effectiveness.
Subgroup analysis identified specific economic benefits of HAIC-FO in patients with Vp4 PVTT and high tumour burden.
Sensitivity analyses confirmed the robustness of the results with a 99.9% probability of cost-effectiveness.
This study focused on the Chinese healthcare payer perspective, which may limit generalisability to regions with differing economic conditions.
Introduction
Hepatocellular carcinoma (HCC) is the fourth most common cause of cancer-related mortality worldwide.1 In developing nations, particularly in China, most patients develop advanced HCC.2 Prior to 2017, sorafenib was the recommended first-line systemic therapy for HCC; lenvatinib was approved as a first-line treatment option in 2018.3 Following IMbrave 150 trial results published in 2020,4 Chinese and international guidelines have endorsed atezolizumab plus bevacizumab (T+A) as the preferred first-line regimen for patients with unresectable HCC and no prior systemic treatment.5–8 However, current evidence suggests that T+A may not be cost-effective compared with sorafenib.9–12
Although hepatic artery infusion chemotherapy (HAIC) is not universally recognised as a well-established treatment regimen, it is effective and commonly used in treating advanced HCC in East Asian countries.5 The recent FOHAIC-1 trial, a phase III trial to verify the survival benefit of HAIC compared with sorafenib, demonstrated that HAIC with fluorouracil, leucovorin and oxaliplatin (HAIC-FO) improved clinical outcomes compared with sorafenib in advanced HCC.13 Median overall survival was 13.9 months for HAIC-FO versus 8.2 months for sorafenib (HR, 0.408; p<0.001), and median progression-free survival was 7.8 months versus 4.3 months, respectively (HR, 0.451; p<0.001). Despite these reported clinical benefits of HAIC-FO in advanced HCC, its cost-effectiveness has not been thoroughly evaluated.
Concerns about the economic value of the T+A regimen persist, prompting exploration of alternative therapies with improved cost-effectiveness. This study compared the cost-effectiveness of HAIC-FO and sorafenib in Chinese patients with advanced HCC.
Materials and methods
FOHAIC-1 trial
The Institutional Review Board approved this study (IRB No.SB5010-2017-015). All participants provided informed consent. Patients and the public were not involved in the design, conduct, reporting or dissemination of our research plans. The trial, carried out at a medical institution in China, was a phase 3 randomised study comparing the therapeutic effects of HAIC-FO and sorafenib in advanced HCC.13 The primary inclusion criterion was a dominant liver mass, with additional criteria detailed in ClinicalTrials.gov (registration ID: 03164382). From 2017 to 2020, a total of 262 eligible patients were randomised into two groups: 130 received HAIC-FO, and 132 received sorafenib therapy (1:1 ratio; figure 1). Among these, 89.3% had hepatitis B virus (HBV) infection, and 82.8% exhibited macrovascular invasion. In the HAIC-FO arm, the regimen consisted of sequential infusions of oxaliplatin (130 mg/m2), leucovorin (200 mg/m2), fluorouracil (400 mg/m2) and fluorouracil (2400 mg/m2) administered via catheter every 3 weeks. The HAIC-FO procedure and treatment-associated adverse events (AEs) have been previously described.13–15 Patients in the sorafenib control group received 800 mg of sorafenib orally per day, divided into two doses, with dosage adjustments as needed. Subsequent treatments are detailed in online supplemental table S1.
Supplemental material
Study flow diagram. ECOG-PS, Eastern Cooperative Oncology Group performance status; HAIC-FO, hepatic artery infusion chemotherapy with fluorouracil, leucovorin and oxaliplatin; TACE, transarterial chemoembolisation.
Economic evaluation using Markov model
TreeAge 2011 software was adopted in this study, following the Consolidated Health Economic Evaluation Reporting Standards reporting guidelines for constructing a Markov model for cost-effectiveness analysis.16 In this model, the health status was categorised into three types: stable, progressive and death. Patients underwent HAIC-FO or sorafenib therapy in the stable disease stage, while during disease progression, they received second-line therapy until death. The time horizon of the model was 42 months. This study deemed HAIC-FO to be economically viable if the incremental cost-effectiveness ratio (ICER) was under a certain willingness-to-pay (WTP) threshold. Specifically, WTP thresholds refer to WTP per quality-adjusted life years (QALYs). Based on previous research, we set WTP thresholds in China to $30 492 per QALY.17 Table 1 and online supplemental table S2 provide details of the model parameters and their corresponding sources. When patients received HAIC-FO treatment, hospitalisation was necessary, and they were responsible for covering the hospitalisation cost. However, if the patient received sorafenib treatment, hospitalisation was not required, and the cost did not increase. Furthermore, when patients received HAIC-FO treatment, they needed to pay an additional fee apart from the hospitalisation cost. The present study assigned QALY values as follows: 0.76 in the absence of disease progression, 0.68 with disease progression and 0 on death.17 This research considered grade ≥3 AEs—including elevated total bilirubin, hypertension, fatigue, neutropenia, thrombocytopenia and elevated aspartate aminotransferase (AST)/alanine transaminase—with an incidence exceeding 1% as documented in Lyu’s study.13 To simplify the modelling, we assumed that all AEs occurred during the first treatment cycle and that subjects could experience more than one AE simultaneously.
Markov inputs in cost-effectiveness analysis
Statistical analysis
During the cost-effectiveness analysis, we performed a sensitivity analysis to evaluate how uncertainties in treatment effectiveness, costs and utilities influenced the final ICER. Model parameters were derived from relevant literature, with values varied by ±20% from baseline to define parameter ranges. The discount rate was set at 0%–5%. In Monte Carlo simulations, 1000 iterations were conducted, and each key parameter was assigned a probability distribution (eg, costs modelled using gamma distributions and utilities modelled using beta distributions).
Patient and public involvement
Patients and the public were not involved in the design or conduct of this study.
Results
Base case results
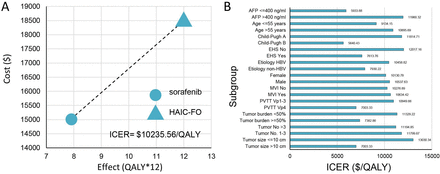
In the base-case analysis, the HAIC-FO arm incurred a total cost of $18 470.98 and yielded 1.0 QALY. The sorafenib group had a total cost of $15 011.73, and yielded 0.66 QALY (table 2). Compared with sorafenib, HAIC-FO resulted in an ICER of $10 235.56 per QALY, which was below the WTP threshold in China (figure 2A).
Base-case and subgroup analysis results. (A) Incremental cost-effectiveness ratio (ICER) for hepatic artery infusion chemotherapy with fluorouracil, leucovorin and oxaliplatin (HAIC-FO) compared with sorafenib: $10 235.56 per quality-adjusted life year (QALY). (B) Subgroup analysis of cost-effectiveness. AFP, alpha-fetoprotein; EHS, extrahepatic spread; HBV, hepatitis B virus; ICER, incremental cost-effectiveness ratio; MVI, macrovascular invasion; PVTT, portal vein tumour thrombus; QALYs, quality-adjusted life-years.
Cost-effectiveness results
Subgroup analyses revealed that the ICERs for HAIC-FO compared with sorafenib were below the WTP threshold across all analysed subgroups. These included tumour size (>10 vs ≤10 cm), tumour number (1–3 vs >3), tumour burden (≥50% vs <50%), portal vein tumour thrombosis (Vp4 vs Vp1–3), macrovascular invasion (yes vs no), gender (male vs female), aetiology (HBV vs non-HBV), extrahepatic spread (yes vs no), Child-Pugh score (B vs A), age (>55 vs ≤55 years) and alpha-fetoprotein level (>400 vs ≤400 ng/mL) (figure 2B). Specifically, the ICER was $7003.33 per QALY for patients with Vp4 portal vein tumour thrombus and $7382.86 per QALY for those with a high tumour burden.
One-way sensitivity analysis
We conducted a one-way sensitivity analysis to identify key parameters influencing the cost-effectiveness comparison between HAIC-FO and sorafenib. Figure 3A presents a tornado diagram illustrating variations in the cost-effectiveness of HAIC-FO versus sorafenib across modelled parameters. The tornado diagram confirmed that HAIC-FO maintained an ICER below the $30 492 per QALY WTP threshold across all parameters compared with sorafenib.
Sensitivity analysis. (A) Tornado diagram of the one-way sensitivity analysis for the incremental cost-effectiveness ratio (ICER) comparing hepatic artery infusion chemotherapy with fluorouracil, leucovorin and oxaliplatin (HAIC-FO) and sorafenib, with parameters ranked by their impact on ICER variability. (B) Probabilistic sensitivity analysis (1000 Monte Carlo simulations) demonstrating the cost-effectiveness of HAIC-FO. Dots below the line represent simulations where the cost per quality-adjusted life year (QALY) gained was below the willingness-to-pay (WTP) threshold. Input parameters and distributions are detailed in table 1. (C) Cost-effectiveness acceptability curves for HAIC-FO and sorafenib across varying WTP thresholds. OS, overall survival; PFS, progression-free survival.
Parameters related to AEs had the smallest impact on ICER variability, with fluctuations of less than $11.50 per QALY. Consequently, AE parameters were excluded from figure 3A.
Probabilistic sensitivity analysis
Based on Monte Carlo analysis, the HAIC-FO strategy was cost-effective in 99.9% simulations in China (figure 3B). As shown in figure 3C, probabilistic sensitivity analysis demonstrated that as the WTP threshold per incremental QALY increased, the proportion of modelled scenarios favouring HAIC-FO as the cost-effective strategy rose correspondingly, while the proportion favouring sorafenib declined. At a WTP threshold of $10 000 per QALY, HAIC-FO was the preferred strategy in 48.9% of simulations; this increased to 97.2% at $20 000, 99.9% at $30 000 and 100.0% at $40 000.
Discussion
The cost of HCC treatment constitutes a substantial portion of cancer-related healthcare expenditure, underscoring the need to evaluate health-economic implications of HAIC-FO. This study employed a Markov model to compare the cost-effectiveness of HAIC-FO and sorafenib in patients with advanced HCC. Our findings indicate that HAIC-FO is a cost-effective therapeutic option from the perspective of Chinese payers. These results provide valuable insights for policymakers, clinicians and patients regarding the role of HAIC-FO in HCC management from a health-economic perspective (figure 4).
Schematic diagram of the study. AFP, alpha-fetoprotein; ECOG, Eastern Cooperative Oncology Group; EHS, extrahepatic spread; HAIC-FO, hepatic artery infusion chemotherapy with fluorouracil, leucovorin, and oxaliplatin; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; ICER, incremental cost-effectiveness ratio; MVI, macrovascular invasion; PVTT, portal vein tumour thrombus; QALY, quality-adjusted life year.
Recent clinical benefits offered by HAIC-FO have garnered wide attention from physicians and patients.18 However, few cost-effectiveness studies comparing HAIC-FO or HAIC-FO-based therapy with sorafenib for advanced HCC have been published, contributing to uncertainty in healthcare decision-making.19–21 Our findings demonstrate that HAIC-FO is a cost-effective therapeutic alternative to sorafenib for advanced HCC, a conclusion supported by results across a wide range of parameters. Li et al previously reported that combining HAIC-FO with sorafenib was not cost-effective compared with sorafenib monotherapy for HCC with portal vein invasion.17 However, this conclusion requires cautious interpretation, as their model assumed continuous HAIC-FO administration until disease progression. The authors modelled HAIC-FO treatment over 8 years—a duration far exceeding real-world clinical practice, where HAIC-FO is typically administered every 3 weeks for 6–8 months (eight sessions). This discrepancy suggests potential overestimation of HAIC-FO costs in their analysis. For instance, in patients surviving beyond 8 months, the model used by Li et al would inflate HAIC-FO-related expenses. In contrast, our study limited HAIC-FO treatment to six to eight cycles, aligning with clinical trials protocols and real-world clinical guidelines.
HAIC was cost-effective compared with sorafenib when AEs—including neutropenia, elevated AST and thrombocytopenia—were incorporated into the Markov model for ICER calculation. One-way sensitivity analysis further confirmed that AEs had minimal impact on cost-effectiveness outcomes (excluded from tornado plot). Despite their limited influence on cost-effectiveness, AEs retain clinical significance as they directly affect patient health-related quality of life and treatment adherence. Kudo et al22 reported higher rates of ≥grade 3 AEs with HAIC-based therapy; however, these were manageable through treatment interruption or dose reduction. Future research should prioritise evaluating the efficacy, safety and economic benefits of HAIC combination therapies, which are emerging as a key focus in HCC management.
While our findings indicate HAIC-FO as a more cost-effective option than sorafenib, it is important to acknowledge that sorafenib—an orally administered medication—offers advantages in convenience and independence from medical facilities or specialised personnel. Therefore, given regional disparities in healthcare infrastructure across China,23 interventional procedures like HAIC-FO may be less feasible in areas with limited equipment or insufficient trained staff compared with oral or intravenous therapies. Furthermore, the National Healthcare Security Administration of China has actively reduced drug costs through centralised procurement and volume-based pricing.24 Our analysis accounted for potential drug price fluctuations and confirmed the cost-effectiveness superiority of HAIC-FO over sorafenib under these dynamic conditions.
Several limitations of this study should be acknowledged. First, most patients presented high liver tumour burden at initial diagnosis, which is frequently linked to HBV infection. Notably, sorafenib demonstrates limited efficacy in HBV infection-related HCC but significantly improves survival in hepatitis C virus (HCV)-related HCC.25 Consequently, the health-economics evaluation of HAIC-FO and sorafenib remains uncertain in regions where HCC aetiology differs (eg, HCV infection or alcohol use). Additionally, this single-centre study was conducted in China, limiting generalisability to regions with distinct healthcare systems, treatment protocols or economic conditions.26 Second, chemotherapeutic regimens exhibit considerable heterogeneity.13 27–29 Further research is needed for rigorously evaluating the cost-effectiveness and safety of diverse HAIC strategies across populations. Third, Markov modelling carries inherent limitations related to assumptions and input data quality. However, our findings remained robust across sensitivity analysis, suggesting minimal impact from alternative inputs. Finally, future studies should compare HAIC-FO cost-effectiveness with other advanced HCC therapies—including emerging systemic treatments—to enable comprehensive economic evaluations.
In summary, the HAIC-FO strategy demonstrates greater cost-effectiveness than sorafenib for advanced HCC in Chinese patients, particularly among individuals with Vp4 portal vein tumour thrombus or high tumour burden.
Data availability statement
Data are available in a public, open access repository. The datasets generated or analysed during the study are available in the Research Data Deposit repository (https://www.researchdata.org.cn/Search.aspx?k=RDDA2021002021).
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants. The Sun Yat-sen University Cancer Center review board approved the study (IRB No.SB5010-2017-015). Every participant provided informed consent. Participants gave informed consent to participate in the study before taking part.
References
Footnotes
Q-FC, XJ, YH and SC contributed equally.
Contributors Conceptualisation: Q-FC and MZ. Data curation: Q-FC and XJ. Formal analysis: Q-FC, XJ, YH and SC. Funding acquisition: Q-FC, NL and MZ. Investigation: Q-FC, XJ, YH and SC. Methodology: Q-FC and XJ. Project administration: MZ. Resources: MZ. Software: MZ. Supervision: MZ. Validation: Q-FC, XJ, YH and SC. Visualisation: Q-FC and XJ. Writing–original draft: Q-FC and XJ. Writing–review and editing: MZ is the guarantor.
Funding Supported by the National Natural Science Foundation of China (No. 82402403 and No. 82072022) and Guangdong Basic and Applied Basic Research Foundation (No. 2025A1515011330).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.