Article Text
Abstract
Introduction Acute kidney injury (AKI) is a major complication after cardiac surgery and is associated with postoperative morbidity and mortality. Currently, no effective therapy exists to reduce the incidence of postoperative AKI. Sodium-glucose cotransporter-2 (SGLT2) inhibitors are effective in reducing AKI in outpatient settings for patients with chronic kidney disease. We hypothesised that perioperative SGLT2 inhibition will also reduce AKI incidence after cardiac surgery according to Kidney Disease: Improving Global Outcomes (KDIGO) criteria.
Methods and analysis We designed a multicentre, randomised, placebo-controlled, triple-blinded, superiority trial. A total of 784 patients, aged above 18 years, undergoing cardiac surgery will be included with stratification for sex and type 2 diabetes in a 1:1 ratio. Patients will receive either dapagliflozin 10 mg or placebo from the day before until 2 days after surgery. Serum creatinine will be measured preoperatively and daily for the first 7 days after the operation, and urine output will be measured until the urinary catheter is removed. The primary outcome is the incidence of postoperative AKI according to the KDIGO criteria.
Ethics and dissemination The medical ethics committee of the Amsterdam University Medical Centre (UMC) and the Dutch competent authority approved the study protocol (currently, version 9, 19 January 2024). This is an investigator-initiated study. The Amsterdam UMC, as sponsor, retains ownership of all data and publication rights. After completion of the trial, results will be disseminated to participants, patient societies and physicians via a network meeting and digital newsletter. Results will be submitted for publication in a peer-reviewed international medical journal and presented on (inter)national congresses.
Trial registration number Clinicaltrials.gov identifier: NCT05590143.
- Cardiac surgery
- Randomized Controlled Trial
- acute kidney injury
This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
A randomised controlled trial using sodium-glucose cotransporter-2 inhibitors to prevent acute kidney injury (AKI) after cardiac surgery.
Multicentre, randomised, triple-blind and placebo-controlled.
An easily clinically implementable intervention starting only 1 day prior to the operation.
Repurposing of a widely-used and safe kidney protective drug.
As postoperative AKI is the primary outcome in this study, further research is required to investigate the longer-term impact.
Introduction
Annually, more than 2 million cardiac surgeries are performed worldwide, and the incidence of cardiac surgery-associated acute kidney injury (CSA-AKI) is reported to be up to 70%.1 Currently, no evidence-based preventive options are available.2 The reported incidence varies considerably between countries and centres, which may depend on standards of surgery as well as perioperative care, selected patient population, type of surgery and, perhaps most importantly, disparities in AKI definition and monitoring. The majority (71%) of diagnosed cases of CSA-AKI are classified as stage 1, according to Kidney Disease: Improving Global Outcomes (KDIGO) criteria, which include an increase in serum creatinine of ≥0.3 mg/dL within 48 hours, ≥ 1.5 times baseline within 7 days or urine output <0.5 mL/kg/h for 6–12 hours and resolve without treatment or medical intervention.3 However, 12% of cases fall into stage 3, potentially necessitating renal replacement therapy for the management of AKI.3 CSA-AKI is independently associated with higher postoperative mortality compared with patients without AKI: 3 times higher mortality for patients with stage 1 AKI and up to 37 times higher for patients requiring dialysis (stage 3 AKI). Patients with AKI also have enhanced length of stay in the intensive care unit (ICU) and hospital and marked increments in cost of care, ranging from 1.21 times higher costs for stage 1 AKI to 2.74 for stage 3 AKI requiring dialysis.2 4 In addition, patients with only stage 1 AKI have a higher probability of developing chronic kidney disease (CKD) in the years following surgery, as compared with individuals without postoperative AKI.5 The risk of death associated with AKI remains increased for up to 10 years after cardiac surgery, even for those with complete recovery of kidney function.4 6 It is evident from these data that CSA-AKI represents a major unmet medical need posing a burden on patients and society.
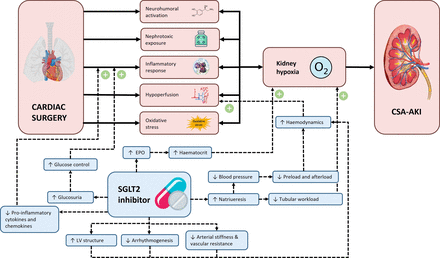
Despite several attempts with a variety of pharmacological interventions, including vasodilators, diuretics, analgesics, antioxidants, cholesterol-lowering and anti-inflammatory drugs, no successful interventions have been identified that prevent CSA-AKI.7 8 However, sodium-glucose cotransporter-2 (SGLT2) inhibitors are promising new drugs to prevent CSA-AKI. SGLT2 inhibitors were initially developed for their glucose-lowering effects by inducing glucosuria.4 In addition, they have obtained a prominent place in clinical practice as they reduced progression of kidney disease in patients with CKD with or without type 2 diabetes (T2D) in large outcome trials.9 Moreover, in these trials, SGLT2 inhibitors reduced AKI incidence (HR 0.66, 95% CI 0.54 to 0.80), even though it was assessed as a safety endpoint in most trials.6 The mechanisms by which SGLT2 inhibitors prevent acute or chronic kidney injury are still the subject of research. SGLT2 inhibitors reduce estimated glomerular filtration rate and glomerular pressure, thereby preventing kidney damage.10 Lowering glomerular hyperfiltration reduces kidney oxygen consumption and may thus ameliorate kidney hypoxia, and since oxygen deprivation is a major contributor to CSA-AKI, this might protect against injury.11 Furthermore, SGLT2 inhibitors improve haemodynamics and exhibit anti-inflammatory properties.12 13 Preclinical data have shown that SGLT2 inhibitors reduced renal ischaemia-reperfusion injury.14–16 An overview of the proposed advantages of SGLT2 inhibitors in reducing CSA-AKI is shown in figure 1.
Overview of the proposed advantages of SGLT2 inhibitors in reducing CSA-AKI. CSA-AKI, cardiac surgery-associated acute kidney injury; EPO, erythropoietin; LV, left ventricle; SGTL2, sodium-glucose cotransporter-2.
We conducted a pilot trial to test the feasibility and kidney protection of SGLT2 inhibitors in the context of cardiac surgery, the Metabolic and Renal outcomes in Cardiac sUrgery patients Receiving SGLT2 Inhibitors (MERCURI), at the Amsterdam University Medical Centre (UMC). This single-centre, open label, randomised, phase IV clinical trial included 55 patients and showed a significant reduction in AKI incidence of 46.7% (95% CI −69.7 to −23.6, p=0.001), comparing the use of an SGLT2 inhibitor (empagliflozin) with the untreated control group.17
The current MERCURI-2 trial investigates the effect of perioperative SGLT2 inhibition on the incidence of AKI in patients undergoing cardiac surgery.
Methods
The MERCURI-2 trial (clinicaltrials.gov identifier: NCT05590143) is a multicentre randomised triple-blind placebo-controlled (1:1) superiority trial in patients undergoing cardiac surgery. The trial is designed to evaluate the effect of dapagliflozin 10 mg once daily, compared with placebo, added to standard of care, on the incidence of CSA-AKI. This is an investigator-initiated study, with Amsterdam UMC as sponsor. The study protocol was approved by the Medical Ethics Committee of the Amsterdam UMC and by the Competent Authorities according to national and international regulations (METC 2022.0795 and EudraCT number 2022-002453-25). The first patient was included in June 2023. The trial has an estimated duration of 3 years.
Trial population
Our aim is to enrol 784 adult patients (aged above 18 years) scheduled to undergo elective cardiac surgery. Detailed exclusion criteria are listed (table 1). Patients with type 1 diabetes (T1D) are excluded because of the risk of SGLT-2 inhibitor-associated euglycaemic diabetic ketoacidosis (euDKA) in this patient population. For the same reason, patients with a history of DKA are excluded. Furthermore, patients with T2D with body mass index (BMI) <25 kg/m2 using multiple daily insulin injections are excluded as they phenotypically resemble T1D, placing them at higher risk for euDKA. Patients with a systolic blood pressure below 100 mm Hg at the time of inclusion are excluded due to the possible blood pressure lowering characteristics of dapagliflozin. Furthermore, patients currently treated with SGLT2 inhibitors or with known or suspected allergy to dapagliflozin are excluded from participation.
Inclusion and exclusion criteria of the MERCURI-2 trial
Study design
Potentially eligible patients who are scheduled for cardiac surgery will be contacted via telephone, during preoperative hospital admission or during a preoperative hospital visit. Patients will receive both written and oral information (online supplemental file 1). After informed consent is obtained and signed, baseline characteristics such as age, sex, ethnicity, BMI, blood pressure, American Society of Anesthesiologist physical status, medical history and medication use will be collected. The most recent serum creatinine and glycated haemoglobin A1c, if measured as part of standard medical care before surgery, will be recorded. Patients will subsequently be randomised to dapagliflozin or placebo. Patients will take the first dapagliflozin 10 mg or placebo on the day before surgery, between 15.00 and 20.00. The second dosage will be taken on the morning before surgery. The remainder dosages will be taken the first and second day after surgery. In case the surgery is postponed, an additional dose will be administered. Patients with T2D on glucose lowering therapy will be advised on how to adjust their current diabetes treatment to prevent potential hypoglycaemia (figure 2).
Supplemental material
Treatment algorithm for adjustment of glucose lowering therapy in patients with T2D. DPP4, dipeptidyl peptidase IV; GLP-1 RA, glucagon like peptide-1 receptor agonist; HbA1c, haemoglobin A1c; IU, international unit; SU, sulfonylureas; T2D, type 2 diabetes.
Serum creatinine will be measured daily until day 7 or until discharge from hospital. We aim to collect an additional blood sample from 20% of the participants, both on the day before and after surgery, for potential future analyses. Urine output will be reported as measured during routine medical care, until the urinary catheter is removed. Complications (online supplemental table 1) and ICU and hospital length of stay will be registered until 30 days after surgery. After 30 days, patients will receive a questionnaire measuring patient-reported quality of life and disability with questions from the WHO Disability Assessment Schedule 2.0 (WHO-DAS 2.0), Days at Home in first 30 days (DAH30) and 5-level EuroQol 5-dimensions (EQ5D5L). Data will be collected using Castor EDC (Amsterdam, Netherlands), a good clinical practice compliant data management system.18 The study design is summarised in figure 3.
Supplemental material
Summary of the study flow of the MERCURI-2 trial. AF, atrial fibrillation; DAH30, days at home in first 30 days; ICU, intensive care unit; MACE, major adverse cardiovascular events; MAKE, major adverse kidney events; OR, operation room; QoRquality of recovery; SGLT2, sodium-glucose cotransporter-2.
Randomisation
After inclusion, patients will be randomised by Castor EDC. Block randomisation with computer-generated blocks of 4, 6 or 8 patients will be used. The block size will be unknown to the researchers. Randomisation is stratified for sex, presence of T2D and study site.
Allocation concealment and blinding
Investigators will register the patient in Castor EDC. The randomisation outcome will be exclusively visible to the trial pharmacy. Treatment allocation of a patient will only be disclosed in case of a suspected unexpected serious adverse reaction (SUSAR). Study treatment is provided in identical boxes, each containing either six overencapsulated tablets of dapagliflozin or placebo. To ensure blinding, the original 10 mg dapagliflozin (AstraZeneca, Södertälje, Sweden) tablets will be overencapsulated by the good manufacturing practice (GMP) certified trial pharmacy at Amsterdam UMC. The capsules will be filled with microcrystalline cellulose PH102. The matching placebo capsules will solely contain microcrystalline cellulose PH102. Patients, healthcare providers and investigators will be blinded to group allocation until database lock.
Outcomes and outcome adjudication
The primary outcome of this study is the incidence of AKI according to the KDIGO criteria (table 2) based on serum creatinine and urine output. Serum creatinine levels and urine output will be reviewed by a second investigator and the study monitor to ensure primary outcome adjudication. Other secondary kidney-related outcome parameters include the difference in the primary outcome between men and women, incidence of individual AKI stages according to KDIGO criteria, the maximum change of creatinine postoperatively compared with the baseline creatinine preoperatively, the postoperative creatinine course, the number of patients with persistent kidney failure (defined as creatinine concentration ≥200% of baseline creatinine concentration after 30 days), the number of patients requiring dialysis and the number of patients who died due to a primary kidney-related cause. The cardiac-related secondary outcomes include the incidence of de novo postoperative atrial fibrillation, registered on a 12-lead ECG, the maximum postoperative creatine kinasemyocardial band (CK-MB) or troponin concentrations, the number of patients who developed new onset cardiac arrhythmia, new myocardial infarctions or cerebrovascular accident confirmed by CT scan within the first 30 days postoperatively, as well as the number of patients who required an extended hospital stay due to heart failure, needed readmission for heart failure or died from a primary cardiac cause. Furthermore, length of stay in the ICU and the hospital measured in days and all-cause mortality were collected (table 3). Complications within the first 30 days after surgery (online supplemental table 1) are retrieved from the electronic patient file and collected. The outcomes of the patient-reported quality of life and disability measured with three questionnaires, DAH30,19 WHO-DAS 2.020 and EQ5D5L,21 will be evaluated across the treatment groups. Safety outcomes of SGLT2 inhibitors such as genital mycotic infections, DKA and hypoglycaemia will be assembled and compared between the treatment groups.
Definition and staging of acute kidney injury according to kidney disease: improving global outcomes criteria
Recorded secondary and exploratory outcomes
Study oversight and organisation
The core team of the MERCURI-2 investigator group consists of three principal investigators from the Amsterdam UMC and the trial coordinator. This team is responsible for the design of the study protocol and progress of the trial. The core team will draft the final report that will be approved by all MERCURI-2 investigators. The trial is conducted under the supervision of an independent Data and Safety Monitoring Board (DSMB), consisting of two clinical experts and an epidemiologist. An independent statistician will present unblinded data to the DSMB. The DSMB reviews the incidence of AKI at two predefined intervals (after inclusion of 200 and 400 participants) to assess safety and efficacy outcomes. The DSMB can advise to stop the trial if patient safety is at risk or in case of overwhelming efficacy.
The study will be monitored by the Clinical Research Unit from the Amsterdam UMC. Each involved study site will receive a baseline and close-out visit and additional visits after the inclusion of 10 and 100 participants during the study. The monitor will approve informed consent papers, verify the serious adverse events (SAEs) and check data collection and quality of registration.
Regarding SAEs, the sponsor will yearly present an overview of the SAEs via Clinical Trials Information System (CTIS). SUSARs will be reported earlier via EudraVigilance depending on the seriousness of the reaction and will be as follows: in the case of fatal or life-threatening SUSARs, as soon as possible and in any event not later than 7 days after the sponsor became aware of the reaction; in the case of non-fatal or non-life-threatening SUSARs, not later than 15 days after the sponsor became aware of the reaction and in the case of SUSARs which were initially considered to be non-fatal or non-life-threatening but which turn out to be fatal or life-threatening. as soon as possible and in any event not later than 7 days after the sponsor became aware of the reaction being fatal or life-threatening.
Sample size calculation
Sample size is based on Fisher’s exact test, with an expected AKI incidence in the placebo group of 22%, based on previous cohorts,2 taking a conservative estimation. A relative risk of 0.64 based on AKI reduction by SGLT2 inhibitors in previous trials6 translates into an absolute risk reduction of 7.9% and incidence in the intervention group of 14.1%. The required total sample size to find such a difference with two-sided alpha of 0.05 and 80% power is 784. We therefore aim to include 392 patients per arm.
Achieve sample size
Based on previous trials and the short trial duration per patient, we expect a low number of drop-outs. If a drop-out occurs, for example due to surgery cancellation or withdrawal of informed consent, dropouts will be replaced to maintain sufficient statistical power.
Statistical analyses
The baseline characteristics of all subjects, per treatment group, will be outlined in a table describing variables such as demographic variables, weight, length, relevant medical history and current medication use. Continuous data will be presented as mean with SD or as median with IQR, depending on the distribution of the data. Distribution of data will be assessed with histograms, Q-Q plots and the Shapiro-Wilk test. We expect a small percentage of missing data due to the in-hospital setting and short duration of the study.
Primary study outcome
Statistical analyses will be based on an intention-to-treat approach. The main analysis will concern the comparison of the incidence of AKI between both groups. The disparity in AKI incidence will be evaluated using the Fisher’s exact test. In addition, the risk difference of AKI between both groups, along with the associated 95% CIs, will be presented.
Secondary study outcomes
Heterogeneity of treatment effect between men and women will be studied for the primary outcome by examining a treatment-by-sex interaction effect in a logistic regression model with treatment and sex as main effects. Similarly, the heterogeneity of treatment effect in patients with and without T2D will be studied using the same approach. Treatment effect estimates, along with their corresponding 95% CIs, will be reported for each specific subgroup. Other secondary outcomes will be presented as number of events and analysed with the Fisher’s exact test. Continuous data will be presented as mean with SD and differences will be assessed employing the Student’s t-test. Generalised mixed-effect models will be constructed to examine potential differences in the repeated measurements. Predefined post hoc analysis with regression analyses will be conducted to estimate the influence of age, BMI, baseline kidney function and the presence of heart failure on the treatment effect and their possible interactions on the primary outcome. A 2-sided p value <0.05 will be considered statistically significant. Statistical uncertainty will be expressed in 2-sided 95% CIs.
Ethics and dissemination
All participants will receive a written patient information folder, accompanied by additional oral explanation from the study personnel. A subject screening and enrolment log will only be accessible to study personnel and saved on a secure server. Whenever a patient is enrolled in the study, participation in the trial will be recorded in the electronic patient database, visible for all involved healthcare workers. A subjects’ insurance has been taken out. The study protocol was approved by the Medical Ethics Committee of the Amsterdam UMC (METC 2022.0795). After completion of the trial, results will be disseminated to participants, patient societies and physicians via a network meeting and digital newsletter. Results will be submitted for publication in a peer-reviewed international medical journal and presented on (inter)national congresses.
Planning
The first patient was included in June 2023. The trial has an estimated duration of 3 years. Whenever amendments to the protocol will be made, they will be subjected to the Ethics Committee of the Amsterdam UMC for approval and subsequently communicated to all involved study personnel. As sponsor, the Amsterdam UMC will remain owner of all data and rights to publication. The manuscript will be drafted by the core team and approved by the MERCURI-2 investigators.
Data statement
Full protocol, dataset and data management plan will be available upon reasonable request.
Ethics statements
Patient consent for publication
Acknowledgments
We extend our gratitude to the patient representatives of the Dutch patient societies for heart disease (Harteraad) and kidney disease (Nierpatienten Vereniging Nederland) who were involved in the design phase of the trial.
Footnotes
Collaborators The MERCURI-2 study group: Marcel G Dijkgraaf, Robert JM Klautz, Frederique Paulus, Marcella CA Müller, Rients N de Boer, Jorinde AW Polderman, Thomas GV Cherpanath, Catherine SC Bouman, Alexander PJ Vlaar, Denise P Veelo, Lars IP Snel, Ayla Y Stobbe, Abby Roest, Cato Schmitz, Giel Cox, Hajar Taârnit, Imran Uddin, Lars G Knaap, Merlijn Reijrink, Naomi Chen, Divya PR Tika, Nathalie J Huizing, Tahira El Benhaji, Youri Schut, Michelle Verhagen, Maaike SY Thio, Alice C Pap, Yvonne J Jordens, Henrico JFT Wesselink and Eline F Bruggemans. Marcel G Dijkgraaf: Conceptualization, Methodology, Robert JM Klautz: Conceptualization, Methodology, Frederique Paulus: Conceptualization, Methodology, Marcella CA Müller: Conceptualization, Methodology, Rients N de Boer: Investigation, Jorinde AW Polderman: Conceptualization, Methodology, Thomas GV Cherpanath: Conceptualization, Methodology, Catherine SC Bouman: Conceptualization, Methodology, Alexander PJ Vlaar: Conceptualization, Methodology, Denise P Veelo: Conceptualization, Methodology, Lars IP Snel: Conceptualization, Methodology, Investigation, Ayla Y Stobbe: Investigation, Abby Roest: Investigation, Cato Schmitz: Investigation, Giel Cox: Investigation, Hajar Taârnit: Investigation, Imran Uddin: Investigation, Lars G Knaap: Investigation, Merlijn Reijrink: Investigation, Naomi Chen: Investigation, Divya PR Tika: Investigation, Nathalie J. Huizing: Investigation, Tahira El Benhaji: Investigation, Youri Schut: Investigation, Michelle Verhagen: Investigation, Maaike SY Thio: Investigation, Writing - Review & Editing, Alice C Pap: Investigation, Yvonne J Jordens: Investigation, Henrico JFT Wesselink: Investigation, Eline F Bruggemans: Investigation
Contributors Guarantor: AHH. Authors: MJPO-E: investigation, formal analysis, writing - original draft, NPMdO: investigation, writing - review and editing, EDN: investigation, writing - review and editing, MT: investigation, writing - review and editing, FTFS: investigation, writing - review and editing, BMG: investigation, writing - review and editing, TVS: investigation, writing - review and editing, TCDR: investigation, writing - review and editing, MBG: investigation, writing - review and editing, MFV: investigation, writing - review and editing, JW: investigation, writing - review and editing, LMMvdW: investigation, writing - review and editing, SE: conceptualisation, methodology, writing - review and editing, BP: conceptualisation, methodology, writing - review and editing, JH: conceptualisation, methodology, writing - review and editing, Supervision, Funding Acquisition, DHvR: conceptualisation, methodology, writing - review and editing, Supervision, Funding Acquisition, AHH: conceptualisation, methodology, writing - review and editing, supervision, funding acquisition.
Funding This trial is supported by a grant from The Netherlands Organization for Health Research and Development (ZonMw), programme: Optimal use of Medicines, grant number: 10140022010003.
Competing interests DHvR serves as a consultant and received honoraria from Boehringer Ingelheim and Lilly, Merck, Novo Nordisk, Sanofi and AstraZeneca and has received research operating funds from Boehringer Ingelheim and Lilly Diabetes Alliance, AstraZeneca and Novo Nordisk; all honoraria are paid to his employer (Amsterdam University Medical Centers, location VU University Medical Center). All other authors have no completing interest to declare.
Patient and public involvement The patient representatives of the Dutch patient societies for heart disease (Harteraad) and kidney disease (Nierpatienten Vereniging Nederland) were involved in in the design phase of the trial. Their insights were incorporated into the trial’s planning and execution. They also reviewed the patient information letter and provided advice on how to enhance clarity and comprehensibility. Throughout the trial, these representatives will receive updates on the progress of the trial.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.